Set Up and Utilization of a Three-Dimensional In Vitro Bioreactor System for Human Intestinal Studies and Microbial Co-Cultures
- PMID: 39269316
- PMCID: PMC12065549
- DOI: 10.1002/cpz1.70013
Set Up and Utilization of a Three-Dimensional In Vitro Bioreactor System for Human Intestinal Studies and Microbial Co-Cultures
Abstract
The study of human intestinal physiology and host-microbe interactions is crucial for understanding gastrointestinal health and disease. Traditional two-dimensional cell culture models lack the complexity of the native intestinal environment, limiting their utility in studying intestinal biology. Here, we present a detailed protocol for the set up and utilization of a three-dimensional (3D) in vitro bioreactor system for human intestinal studies and bacterial co-culture. This article outlines the design and assembly of the bioreactor system, scaffold fabrication, bacterial culture techniques, analysis methods, and troubleshooting tips. By providing step-by-step instructions, the goal is to enable other laboratories to utilize physiologically relevant tissue models of the human intestine, incorporating key features, such as nutrient flow, multiple human cell types, 3D architecture, and microbial communities. The incorporation of commensal bacteria into the bioreactor system allows for the investigation of complex host-microbe interactions, providing insight into gastrointestinal health and pathology. This article serves as a comprehensive resource for scientists seeking to advance their understanding of intestinal biology toward the development of novel therapeutic strategies for gastrointestinal disorders. © 2024 Wiley Periodicals LLC. Basic Protocol 1: Scaffold design Basic Protocol 2: Intestinal cell culture: Caco2 cells Basic Protocol 3: Intestinal cell culture: organoids Basic Protocol 4: Bioreactor design and set up Basic Protocol 5: Bacteria in 3D bioreactor set up Basic Protocol 6: Bacteria and drug dosing.
Keywords: 3D silk scaffolds; bacteria; bioreactor; intestinal epithelium; organoids.
© 2024 Wiley Periodicals LLC.
Conflict of interest statement
Figures
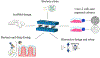


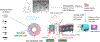
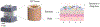

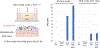


References
-
- Aziza A, Mahmoud R, Zahran E, & Gadalla H (2020). Dietary supplementation of guanidinoacetic acid improves growth, biochemical parameters, antioxidant capacity and cytokine responses in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol, 97, 367–374. doi: 10.1016/j.fsi.2019.12.052 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

