pH-sensing GPR68 inhibits vascular smooth muscle cell proliferation through Rap1A
- PMID: 39269448
- PMCID: PMC11560072
- DOI: 10.1152/ajpheart.00413.2024
pH-sensing GPR68 inhibits vascular smooth muscle cell proliferation through Rap1A
Abstract
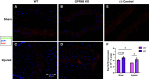
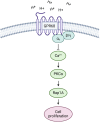
Phenotypic transformation of vascular smooth muscle (VSM) from a contractile state to a synthetic, proliferative state is a hallmark of cardiovascular disease (CVD). In CVD, diseased tissue often becomes acidic from altered cellular metabolism secondary to compromised blood flow, yet the contribution of local acid/base imbalance to the disease process has been historically overlooked. In this study, we examined the regulatory impact of the pH-sensing G protein-coupled receptor GPR68 on vascular smooth muscle (VSM) proliferation in vivo and in vitro in wild-type (WT) and GPR68 knockout (KO) male and female mice. Arterial injury reduced GPR68 expression in WT vessels and exaggerated medial wall remodeling in GPR68 KO vessels. In vitro, KO VSM cells showed increased cell-cycle progression and proliferation compared with WT VSM cells, and GPR68-inducing acidic exposure reduced proliferation in WT cells. mRNA and protein expression analyses revealed increased Rap1A in KO cells compared with WT cells, and RNA silencing of Rap1A reduced KO VSM cell proliferation. In sum, these findings support a growth-inhibitory capacity of pH-sensing GPR68 and suggest a mechanistic role for the small GTPase Rap1A in GPR68-mediated VSM growth control. These results shed light on GPR68 and its effector Rap1A as potential targets to combat pathological phenotypic switching and proliferation in VSM.NEW & NOTEWORTHY Extracellular acidosis remains an understudied feature of many pathologies. We examined a potential regulatory role for pH-sensing GPR68 in vascular smooth muscle (VSM) growth in the context of CVD. With in vivo and in vitro growth models with GPR68-deficient mice and GPR68 induction strategies, novel findings revealed capacity of GPR68 to attenuate growth through the small GTPase Rap1A. These observations highlight GPR68 and its effector Rap1A as possible therapeutic targets to combat pathological VSM growth.
Keywords: GPR68; Rap1A; acidosis; proliferation; vascular smooth muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





Comment in
-
The pH-sensing GPR68 and Rap1A: new kids in the arterial remodeling block.Am J Physiol Heart Circ Physiol. 2024 Nov 1;327(5):H1208-H1209. doi: 10.1152/ajpheart.00671.2024. Epub 2024 Oct 11. Am J Physiol Heart Circ Physiol. 2024. PMID: 39392477 Free PMC article. No abstract available.
References
-
- Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL , et al. 2024 Heart Disease and Stroke Statistics: a report of US and global data from the American Heart Association. Circulation 149: e347–e913, 2024. [Erratum in Circulation 149: e1164, 2024]. doi: 10.1161/CIR.0000000000001209. - DOI - PMC - PubMed
-
- World Health Organization. World Health Statistics 2023: Monitoring Health for the SDGs. Geneva: World Health Organization, 2023.
-
- Holt AW, de Castro Brás LE, Tulis DA. Cyclic nucleotide-driven protein kinase signaling in arterial smooth muscle (patho)physiology. In: Coronary Artery Disease - Causes, Symptoms & Treatments (1st ed.). Columbia, SC: iCONCEPT Press, ; p. 15–51, 2016. ISBN:978-1-922227-92-8.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

