Glucose metabolite methylglyoxal induces vascular endothelial cell pyroptosis via NLRP3 inflammasome activation and oxidative stress in vitro and in vivo
- PMID: 39269632
- PMCID: PMC11399538
- DOI: 10.1007/s00018-024-05432-8
Glucose metabolite methylglyoxal induces vascular endothelial cell pyroptosis via NLRP3 inflammasome activation and oxidative stress in vitro and in vivo
Abstract
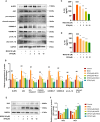
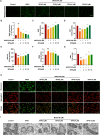
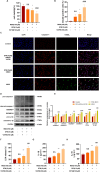
Methylglyoxal (MGO), a reactive dicarbonyl metabolite of glucose, plays a prominent role in the pathogenesis of diabetes and vascular complications. Our previous studies have shown that MGO is associated with increased oxidative stress, inflammatory responses and apoptotic cell death in endothelial cells (ECs). Pyroptosis is a novel form of inflammatory caspase-1-dependent programmed cell death that is closely associated with the activation of the NOD-like receptor 3 (NLRP3) inflammasome. Recent studies have shown that sulforaphane (SFN) can inhibit pyroptosis, but the effects and underlying mechanisms by which SFN affects MGO-induced pyroptosis in endothelial cells have not been determined. Here, we found that SFN prevented MGO-induced pyroptosis by suppressing oxidative stress and inflammation in vitro and in vivo. Our results revealed that SFN dose-dependently prevented MGO-induced HUVEC pyroptosis, inhibited pyroptosis-associated biochemical changes, and attenuated MGO-induced morphological alterations in mitochondria. SFN pretreatment significantly suppressed MGO-induced ROS production and the inflammatory response by inhibiting the NLRP3 inflammasome (NLRP3, ASC, and caspase-1) signaling pathway by activating Nrf2/HO-1 signaling. Similar results were obtained in vivo, and we demonstrated that SFN prevented MGO-induced oxidative damage, inflammation and pyroptosis by reversing the MGO-induced downregulation of the NLRP3 signaling pathway through the upregulation of Nrf2. Additionally, an Nrf2 inhibitor (ML385) noticeably attenuated the protective effects of SFN on MGO-induced pyroptosis and ROS generation by inhibiting the Nrf2/HO-1 signaling pathway, and a ROS scavenger (NAC) and a permeability transition pore inhibitor (CsA) completely reversed these effects. Moreover, NLRP3 inhibitor (MCC950) and caspase-1 inhibitor (VX765) further reduced pyroptosis in endothelial cells that were pretreated with SFN. Collectively, these findings broaden our understanding of the mechanism by which SFN inhibits pyroptosis induced by MGO and suggests important implications for the potential use of SFN in the treatment of vascular diseases.
Keywords: MGO; NLRP3 inflammasome; Pyroptosis; ROS; Sulforaphane.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this article. Figures were created with Bio Render software. Cbiorender.com.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

