Extracellular vesicle-encapsulated miR-30c-5p reduces aging-related liver fibrosis
- PMID: 39269881
- PMCID: PMC11634720
- DOI: 10.1111/acel.14310
Extracellular vesicle-encapsulated miR-30c-5p reduces aging-related liver fibrosis
Abstract
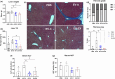
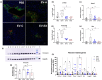
Aging is associated with decreased health span, and despite the recent advances made in understanding the mechanisms of aging, no antiaging drug has been approved for therapy. Therefore, strategies to promote a healthy life in aging are desirable. Previous work has shown that chronic treatment with extracellular vesicles (EVs) from young mice prolongs lifespan in old mice, but the mechanism of action of this effect on liver metabolism is not known. Here we investigated the role of treatment with EVs derived from young sedentary (EV-C) or exercised (EV-EX) mice in the metabolism of old mice and aimed to identify key youthful-associated microRNA (miRNA) cargos that could promote healthy liver function. We found that aged mice treated with either EV-C or EV-EX had higher insulin sensitivity, higher locomotor activity resulting in longer distance traveled in the cage, and a lower respiratory exchange ratio compared to mice treated with EVs from aged mice (EV-A). In the liver, treatment with young-derived EVs reduced aging-induced liver fibrosis. We identified miR-30c in the EVs as a possible youth-associated miRNA as its level was higher in circulating EVs of young mice. Treatment of aged mice with EVs transfected with miR-30c mimic reduced stellate cell activation in the liver and reduced fibrosis compared to EV-negative control by targeting Foxo3. Our results suggest that by delivering juvenile EVs to old mice, we can improve their liver health. Moreover, we identified miR-30c as a candidate for antiaging liver therapy.
Keywords: aging; exercise; extracellular vesicles; fibrosis; miR‐30; microRNAs.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Aravinthan, A. D. , & Alexander, G. J. M. (2016). Senescence in chronic liver disease: Is the future in aging? Journal of Hepatology, 65, 825–834. - PubMed
-
- Boehm, M. , & Slack, F. (2005). A developmental timing microRNA and its target regulate life span in C. elegans . Science, 310, 1954–1957. - PubMed
-
- Castaño, C. , Mirasierra, M. , Vallejo, M. , Novials, A. , & Párrizas, M. (2020). Delivery of muscle‐derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down‐regulation of hepatic FoxO1 in mice. Proceedings of the National Academy of Sciences of the United States of America, 117, 30335–30343. - PMC - PubMed
-
- Chen, X. , Luo, Y. , Zhu, Q. , Zhang, J. , Huang, H. , Kan, Y. , Li, D. , Xu, M. , Liu, S. , Li, J. , Pan, J. , Zhang, L. , Guo, Y. , Wang, B. , Qi, G. , Zhou, Z. , Zhang, C.‐Y. , Fang, L. , Wang, Y. , & Chen, X. (2024). Small extracellular vesicles from young plasma reverse age‐related functional declines by improving mitochondrial energy metabolism. Nature Aging, 4, 814–838. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

