Advanced Quantitative MRI Unveils Microstructural Thalamic Changes Reflecting Disease Progression in Multiple Sclerosis
- PMID: 39270143
- PMCID: PMC11409727
- DOI: 10.1212/NXI.0000000000200299
Advanced Quantitative MRI Unveils Microstructural Thalamic Changes Reflecting Disease Progression in Multiple Sclerosis
Abstract
Background and objectives: In patients with multiple sclerosis (PwMS), thalamic atrophy occurs during the disease course. However, there is little understanding of the mechanisms leading to volume loss and of the relationship between microstructural thalamic pathology and disease progression. This cross-sectional and longitudinal study aimed to comprehensively characterize in vivo pathologic changes within thalamic microstructure in PwMS using advanced multiparametric quantitative MRI (qMRI).
Methods: Thalamic microstructural integrity was evaluated using quantitative T1, magnetization transfer saturation, multishell diffusion, and quantitative susceptibility mapping (QSM) in 183 PwMS and 105 healthy controls (HCs). The same qMRI protocol was available for 127 PwMS and 73 HCs after a 2-year follow-up period. Inclusion criteria for PwMS encompassed either an active relapsing-remitting MS (RRMS) or inactive progressive MS (PMS) disease course. Thalamic alterations were compared between PwMS and HCs and among disease phenotypes. In addition, the study investigated the relationship between thalamic damage and clinical and conventional MRI measures of disease severity.
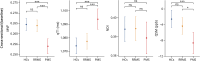
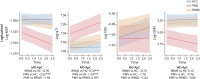
Results: Compared with HCs, PwMS exhibited substantial thalamic alterations, indicative of microstructural and macrostructural damage, demyelination, and disruption in iron homeostasis. These alterations extended beyond focal thalamic lesions, affecting normal-appearing thalamic tissue diffusely. Over the follow-up period, PwMS displayed an accelerated decrease in myelin volume fraction [mean difference in annualized percentage change (MD-ApC) = -1.50; p = 0.041] and increase in quantitative T1 (MD-ApC = 0.92; p < 0.0001) values, indicating heightened demyelinating and neurodegenerative processes. The observed differences between PwMS and HCs were substantially driven by the subgroup with PMS, wherein thalamic degeneration was significantly accelerated, even in comparison with patients with RRMS. Thalamic qMRI alterations showed extensive correlations with conventional MRI, clinical, and cognitive disease burden measures. Disability progression over follow-up was associated with accelerated thalamic degeneration, as reflected by enhanced diffusion (β = -0.067; p = 0.039) and QSM (β = -0.077; p = 0.027) changes. Thalamic qMRI metrics emerged as significant predictors of neurologic and cognitive disability even when accounting for other established markers including white matter lesion load and brain and thalamic atrophy.
Discussion: These findings offer deeper insights into thalamic pathology in PwMS, emphasizing the clinical relevance of thalamic damage and its link to disease progression. Advanced qMRI biomarkers show promising potential in guiding interventions aimed at mitigating thalamic neurodegenerative processes.
Conflict of interest statement
A. Cagol is supported by EUROSTAR E!113682 HORIZON2020, and received speaker honoraria from Novartis; M. Weigel has received research funding by Biogen for developing spinal cord MRI; M. Barakovic is an employee of Hays plc and a consultant for F. Hoffmann-La Roche Ltd; A. Lutti was supported by the Swiss National Science Foundation (Grant Number: 320030_184784) and the ROGER DE SPOELBERCH foundation; P. Calabrese has received honoraria for speaking at scientific meetings, serving at scientific advisory boards, steering committees and consulting activities from Abbvie, Actelion, Almirall, Bayer-Schering, Biogen, BMS, EISAI, Genzyme, Lundbeck, Merck Serono, Novartis, Sanofi-Aventis, Schwabe and Teva, he also receives research Grants from the Swiss Insurance Medicine (SIM) and the Swiss National Research Foundation; J. Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Bayer, Biogen, Celgene, Merck, Novartis, Octave Bioscience, Roche, Sanofi; L. Kappos has received no personal compensation, his institutions (University Hospital Basel/Foundation Clinical Neuroimmunology and Neuroscience Basel) have received and used exclusively for research support: payments for steering committee and advisory board participation, consultancy services, and participation in educational activities from: Actelion, Bayer, BMS, df-mp Molnia & Pohlmann, Celgene, Eli Lilly, EMD Serono, Genentech, Glaxo Smith Kline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, F. Hoffmann-La Roche Ltd, Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics, and Wellmera, and license fees for Neurostatus-UHB products; grants from Novartis, Innosuisse, and Roche; M.P. Sormani received consulting fees from Biogen, Merck, Novartis, Roche, Sanofi, Immunic, Alexion; C. Granziera: The University Hospital Basel (USB), as the employer of C.G., has received the following fees which were used exclusively for research support: (1) advisory board and consultancy fees from Actelion, Genzyme-Sanofi, Novartis, GeNeuro and Roche; (2) speaker fees from Genzyme-Sanofi, Novartis, GeNeuro and Roche; (3) research support from Siemens, GeNeuro, Roche, Cristina Granziera is supported by the Swiss National Science Foundation (SNSF) grant PP00P3_176984, the Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung and the EUROSTAR E!113682 HORIZON2020; all other authors report no competing interests. Go to
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
