IGLON5 Frequency in Idiopathic REM Sleep Behavior Disorder: A Multicenter Study
- PMID: 39270144
- PMCID: PMC11404316
- DOI: 10.1212/NXI.0000000000200311
IGLON5 Frequency in Idiopathic REM Sleep Behavior Disorder: A Multicenter Study
Abstract
Background and objectives: Idiopathic/isolated REM sleep behavior disorder (iRBD) has been strongly linked to neurodegenerative synucleinopathies such as Parkinson disease, dementia with Lewy bodies, and multiple system atrophy. However, there have been increasing reports of RBD as a presenting feature of serious and treatable autoimmune syndromes, particularly IGLON5. This study's objective was to investigate the frequency of autoantibodies in a large cohort of participants with iRBD.
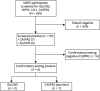
Methods: Participants were enrolled in the North American Prodromal Synucleinopathy cohort with polysomnography-confirmed iRBD, free of parkinsonism and dementia. Plasma samples were systematically screened for the autoantibodies IGLON5, DPPX, LGI1, and CASPR2 using plasma IgG cell-based assay. Positive or equivocal results were confirmed by repeat testing, plus tissue-based indirect immunofluorescence assay for IGLON5.
Results: Of 339 samples analyzed, 3 participants (0.9%) had confirmed positive IGLON5 autoantibodies in the cell-based assay, which were confirmed by the tissue-based assay. An additional participant was positive for CASPR2 with low titer by cell-based assay only (of lower clinical certainty). These cases exhibited a variety of symptoms including dream enactment, cognitive decline, autonomic dysfunction, and motor symptoms. In 1 IGLON5 case and the CASPR2 case, evolution was suggestive of typical synucleinopathy, suggesting the possibility that findings were incidental. However, 2 participants with IGLON5 died before diagnosis was clinically suspected, with a final clinical picture highly suggestive of autoimmune disease.
Discussion: Our finding that nearly 1% of a large iRBD cohort may have a serious but potentially treatable autoantibody syndrome has important clinical implications. In particular, it raises the question of whether autoantibody testing for IGLON-5-IgG should be widely implemented for participants with iRBD, considering the difficulty in diagnosis of autoimmune diseases, their response to treatment, and the potential for rapid disease progression. However, any routine testing protocol will also have to consider costs and potential adverse effects of false-positive findings.
Trial registration information: NCT03623672.
Conflict of interest statement
R. Postuma has received support from the Fonds de Recherche du Quebec - Santé, the Canadian Institutes of Health Research, the Parkinson Society of Canada, the Weston-Garfield Foundation, the Michael J. Fox Foundation, and the Webster Foundation and personal fees from Takeda, Roche/Prothena, Teva Neurosciences, Novartis Canada, Biogen, Boehringer Ingelheim, Theranexus, GE HealthCare, Jazz Pharmaceuticals, Abbvie, Jannsen, Otsuko, Phytopharmics, Inception Sciences, and Curasen. N. Vorasoot has no disclosures. E.K. St. Louis has received support from NIH (NIA, NINDS, and NHLBI), the Michael J. Fox Foundation, Harmony, Inc., and Sunovion, Inc.; A.Y. Avidan has received consultant fees from Avadel, Merck, Takeda, Eisai, Idorsia and Harmony and speaker honoraria from Merck, Eisai, Harmony and Idorsia, A. Pelletier has no disclosures. M.M. Lim has received support from federal, state, and non-profit organizations including Department of Veteran Affairs, Department of Defense, NIH (NIMH, NHLBI, NIA, NCCIH, NINDS, NIGMS, NCATS), NSF, Center for Neuroscience & Regenerative Medicine (Henry Jackson Foundation), Oregon Medical Research Foundation, Collins Medical Trust, Brain & Behavior Foundation (NARSAD), American Sleep Medicine Foundation, Hartford Center of Gerontological Excellence, Pacific Northwest National Laboratory, and Portland VA Research Foundation. M.M. Lim receives compensation as a member of the Scientific Advisory Board for Applied Cognition. J. Elliott has received support from the Department of Veteran Affairs, NIH (NHLBI, NIA, NCCIH), Oregon Medical Research Foundation, Portland VA Research Foundation, Eugene & Clarissa Evonuk Foundation in Environmental Physiology, and American Heart Association. J.-F. Gagnon has received support from the NIH, the Canadian Institutes of Health Research and the Fonds de Recherche du Québec - Santé. Z. Gan-Or has received support from Bial, Bial Biotech, Capsida Biotherapeutics, Handl Therapeutics, Idorsia, Neuron23, Ono Pharmaceutical, Prevail Therapeutics, UCB, and Vanqua Bio. L.K. Forsberg has no disclosures. J.A. Fields has received support from the NIH and is a consultant for Medtronic, Inc. O.A. Ross has no disclosures. W. Singer has no disclosures. D.E. Huddleston has received support from NIH (NIA, NINDS, Department of Veteran Affairs, the American Parkinson's Disease Association Center for Advanced Research, the Emory Udall Parkinson's Disease Research Center, the Emory Lewy Body Dementia Association Research Center of Excellence, the Emory Alzheimer's Disease Research Center, the Michael J. Fox Foundation, the Georgia Research Alliance, the Bumpus Family Foundation, and the McMahon Family. D.L. Bliwise has received support from the NIH and has been a Consultant to CliniLabs, Eisai, Ferring, Huxley, Idorsia and Merck. M. Howell has received research support from the NIH. Dr. Schenck has received a one-time speaker honorarium from Eisai, Inc. J. McLeland has no disclosures. A.A. Davis has received research support from the Department of Defense, NIH (NINDS), and the Michael J. Fox Foundation. S.R. Criswell has received support from the NIH and consulting fees from Abbvie and Sio Gene Therapies. A. Videnovic has received research support from the NIH and the Michael J. Fox Foundation; consultancy fees from Alexion Pharmaceuticals, Biogen, XW Pharma, and Jazz. E.H. During has received support from Jazz Pharmaceuticals, Sanofi, Takeda, Rythm Inc., and the Feldman Foundation CA. M.G. Miglis has received support from Jazz Pharmaceuticals, Embr Wave, and Biohaven Pharmaceuticals; consulting fees from 2nd MD, Infinite MD and Guidepoint LLC; payments for CME lectures from MED-IQ; and royalties from Elsevier Inc. B.F. Boeve has served as an investigator for clinical trials sponsored by Alector, Biogen and Transposon. He serves on the Scientific Advisory Board of the Tau Consortium, which is funded by the Rainwater Charitable Foundation. He receives support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, the Little Family Foundation, and the Ted Turner and Family Foundation. Y.-E.S. Ju has received support from the NIH and the Centene Corporation contract (P19-00559) for the Washington University-Centene ARCH Personalized Medicine Initiative and compensation for consultant activities for Applied Cognition. A. McKeon reports research funding from the National Institutes of Health: RO1NS126227, U01NS120901; has patents issued for GFAP and MAP1B-IgGs and patents pending for PDE10A, Septins-5 and -7, and KLCHL11-IgGs; and has consulted for Janssen and Roche pharmaceuticals, without personal compensation. Go to
Figures
References
-
- Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575-586. doi: 10.1016/S1474-4422(14)70051-1 - DOI - PMC - PubMed