Prognostic clinical and biological markers for amyotrophic lateral sclerosis disease progression: validation and implications for clinical trial design and analysis
- PMID: 39270623
- PMCID: PMC11415817
- DOI: 10.1016/j.ebiom.2024.105323
Prognostic clinical and biological markers for amyotrophic lateral sclerosis disease progression: validation and implications for clinical trial design and analysis
Abstract
Background: With increasing recognition of the value of incorporating prognostic markers into amyotrophic lateral sclerosis (ALS) trial design and analysis plans, there is a pressing need to understand which among the prevailing clinical and biochemical markers have real value, and how they can be optimally used.
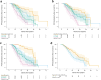
Methods: A subset of patients with ALS recruited through the multi-center Phenotype-Genotype-Biomarker study (clinicaltrials.gov: NCT02327845) was identified as "trial-like" based on meeting common trial eligibility criteria. Clinical phenotyping was performed by evaluators trained in relevant assessments. Serum neurofilament light (NfL) and phosphorylated neurofilament heavy (pNfH), urinary p75ECD, plasma microRNA-181, and an array of biochemical and clinical measures were evaluated for their prognostic value. Associations with functional progression were estimated by random-slopes mixed models of ALS functional rating scale-revised (ALSFRS-R) score. Associations with survival were estimated by log-rank test and Cox proportional hazards regression. Potential sample size savings from adjusting for given biomarkers in a hypothetical trial were estimated.
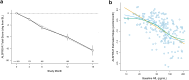
Findings: Baseline serum NfL is a powerful prognostic biomarker, predicting survival and ALSFRS-R rate of decline. Serum NfL <40 pg/mL and >100 pg/mL correspond to future ALSFRS-R slopes of ∼0.5 and ∼1.5 points/month, respectively. Serum NfL also adds value to the best available clinical predictors, encapsulated by the European Network to Cure ALS (ENCALS) predictor score. In models of functional decline, the addition of NfL yields ∼25% sample size saving above those achieved by inclusion of either clinical predictors or ENCALS score alone. The prognostic value of serum pNfH, urinary p75ECD, and plasma miR-181ab is more limited.
Interpretation: Among the multitude of biomarkers considered, only blood NfL adds value to the ENCALS prediction model and should be incorporated into analysis plans for all ongoing and future ALS trials. Defined thresholds of NfL might also be used in trial design, for enrichment or stratified randomisation, to improve trial efficiency.
Funding: NIH (U01-NS107027, U54-NS092091). ALSA (16-TACL-242).
Keywords: ALS clinical trials; Context-of-use; Neurofilament; Prognostic biomarkers.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests MB reports grants from the NIH (U01NS107027, U54NS092091) and the ALS Association (16-TACL-242) in support of this work. He is also an unpaid member of the Board of Trustees for the ALS Association. He has served as a consultant to Alector, Alexion, Annexon, Arrowhead, Biogen, Cartesian, Denali, Eli Lilly, Horizon, Immunovant, Novartis, Roche, Sanofi, Takeda, UCB, and UniQure. EAM reports grants from the NIH (U01NS107027, U54NS092091). He also serves as a consultant to Annexon, Biogen, Bial Biotech, Cortexyme, Chase Therapeutics, Enterin, nQ Medical, Partner Therapeutics, Stoparkinson Healthcare, and UCB. He has served on DSMBs for NeuroSense Therapeutics, Novartis, and Sanofi. AM reports grants from the NIH (U01NS107027). He has also provided consulting services to Roche, Pfizer, and Accure Therapeutics. MLR reports support from grants from the NIH (U01NS107027, U54NS092091) and FightMND. EH has nothing to declare. VL has nothing to declare. DR has nothing to declare. SS has nothing to declare. IM has nothing to declare. YC has nothing to declare. VG currently is an employee of Biohaven Pharmaceutical Inc. JS receives research funding from the NIH < MDA, FSHD Society, Friends of FSH Research, and FSHD Canada. He also serves as a consultant or on scientific advisory boards for Avidity, Fulcrum, Dyne, Armatus, Epic Bio, Roche, Lupin, and Entrada. JH has nothing to declare. RR reports grants from the NIH (U54NS092091) in support of this work. She is also an unpaid member of the Medical Advisory Board of the Association for Frontotemporal Dementias (AFTD) and a paid member of the Scientific Advisory Board of the Kissick Family Foundation FTD Grant Program. CAM reports funding from the ALS Association and the NIH (U54NS092091). LP reports support from the Mayo Clinic Foundation and grants from the National Institute on Aging (5P30AG0062677, U19AG063911) the National Institute of Neurological Disorders and Stroke (U54NS123743, R35NS097273, P01NS084974) and Target ALS Foundation. CM reports grants from the NIH (AG066597, AG076411, AG066152, AG072979, NS109260, NS092091), Department of Defense, and support from the Penn Institute on Aging, Decrane Family PPA Fund, and Newhouse Fund.2. JW reports funding from the NIH (U01NS107027, U54NS092091) and the ALS Association (16-TACL-242) in support of this work. The CReATe Consortium (U54NS092091) is part of the NIH Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. This Consortium is funded through a collaboration between NCATS and the NINDS. This work was also supported by a Clinical Trial Readiness grant (U01NS107027) from NINDS and by a grant from the ALS Association to support the CReATe Biorepository (grant ID 16-TACL-242).
Figures

Update of
-
Prognostic Clinical and Biological Markers for Amyotrophic Lateral Sclerosis Disease Progression: Validation and Implications for Clinical Trial Design and Analysis.medRxiv [Preprint]. 2024 Aug 13:2024.08.12.24311876. doi: 10.1101/2024.08.12.24311876. medRxiv. 2024. Update in: EBioMedicine. 2024 Oct;108:105323. doi: 10.1016/j.ebiom.2024.105323. PMID: 39185513 Free PMC article. Updated. Preprint.
References
-
- Westeneng H.J., Debray T.P.A., Visser A.E., et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018;17(5):423–433. - PubMed
-
- Miller T.M., Cudkowicz M.E., Genge A., et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099–1110. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

