Protofilament-specific nanopatterns of tubulin post-translational modifications regulate the mechanics of ciliary beating
- PMID: 39270640
- PMCID: PMC11466076
- DOI: 10.1016/j.cub.2024.08.021
Protofilament-specific nanopatterns of tubulin post-translational modifications regulate the mechanics of ciliary beating
Abstract
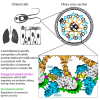
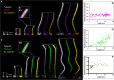
Controlling ciliary beating is essential for motility and signaling in eukaryotes. This process relies on the regulation of various axonemal proteins that assemble in stereotyped patterns onto individual microtubules of the ciliary structure. Additionally, each axonemal protein interacts exclusively with determined tubulin protofilaments of the neighboring microtubule to carry out its function. While it is known that tubulin post-translational modifications (PTMs) are important for proper ciliary motility, the mode and extent to which they contribute to these interactions remain poorly understood. Currently, the prevailing understanding is that PTMs can confer functional specialization at the level of individual microtubules. However, this paradigm falls short of explaining how the tubulin code can manage the complexity of the axonemal structure where functional interactions happen in defined patterns at the sub-microtubular scale. Here, we combine immuno-cryo-electron tomography (cryo-ET), expansion microscopy, and mutant analysis to show that, in motile cilia, tubulin glycylation and polyglutamylation form mutually exclusive protofilament-specific nanopatterns at a sub-microtubular scale. These nanopatterns are consistent with the distributions of axonemal dyneins and nexin-dynein regulatory complexes, respectively, and are indispensable for their regulation during ciliary beating. Our findings offer a new paradigm for understanding how different tubulin PTMs, such as glycylation, glutamylation, acetylation, tyrosination, and detyrosination, can coexist within the ciliary structure and specialize individual protofilaments for the regulation of diverse protein complexes. The identification of a ciliary tubulin nanocode by cryo-ET suggests the need for high-resolution studies to better understand the molecular role of PTMs in other cellular compartments beyond the cilium.
Keywords: Chlamydomonas; U-ExM; cilia motility; cryo-ET; microtubule protofilaments; molecular nanopatterns; motile cilia; tubulin code; tubulin glycylation; tubulin polyglutamylation; tubulin posttranslational modification.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

