A neural basis of choking under pressure
- PMID: 39270654
- PMCID: PMC11791831
- DOI: 10.1016/j.neuron.2024.08.012
A neural basis of choking under pressure
Abstract
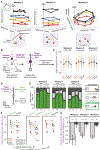
Incentives tend to drive improvements in performance. But when incentives get too high, we can "choke under pressure" and underperform right when it matters most. What neural processes might lead to choking under pressure? We studied rhesus monkeys performing a challenging reaching task in which they underperformed when an unusually large "jackpot" reward was at stake, and we sought a neural mechanism that might result in that underperformance. We found that increases in reward drive neural activity during movement preparation into, and then past, a zone of optimal performance. We conclude that neural signals of reward and motor preparation interact in the motor cortex (MC) in a manner that can explain why we choke under pressure.
Keywords: motivation; motor cortex; movement preparation; paradoxical performance decrement; reaching; reward; reward-mediated performance.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
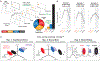
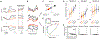
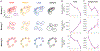
Update of
-
A neural basis of choking under pressure.bioRxiv [Preprint]. 2023 Apr 16:2023.04.16.537007. doi: 10.1101/2023.04.16.537007. bioRxiv. 2023. Update in: Neuron. 2024 Oct 23;112(20):3424-3433.e8. doi: 10.1016/j.neuron.2024.08.012. PMID: 37090659 Free PMC article. Updated. Preprint.
References
-
- Kimble CE, and Rezabek JS (1992). Playing games before an audience: social facilitation or choking. Soc. Behav. Pers 20, 115–120. 10.2224/sbp.1992.20.2.115. - DOI
-
- Ariely D, Gneezy U, Loewenstein G, and Mazar N (2009). Large stakes and big mistakes. Rev. Econ. Stud 76,451–469. 10.1111/j.1467-937X.2009.00534.x. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

