Three classes of propofol binding sites on GABAA receptors
- PMID: 39270821
- PMCID: PMC11490885
- DOI: 10.1016/j.jbc.2024.107778
Three classes of propofol binding sites on GABAA receptors
Abstract
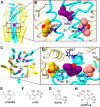
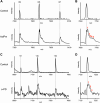
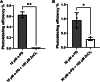
Propofol is a widely used anesthetic and sedative that acts as a positive allosteric modulator of gamma-aminobutyric acid type A (GABAA) receptors. Several potential propofol binding sites that may mediate this effect have been identified using propofol-analogue photoaffinity labeling. Ortho-propofol diazirine (o-PD) labels β-H267, a pore-lining residue, whereas AziPm labels residues β-M286, β-M227, and α-I239 in the two membrane-facing interfaces [β(+)/α(-) and α(+)/β(-)] between α and β subunits. This study used photoaffinity labeling of α1β3 GABAA receptors to reconcile the apparently conflicting results obtained with AziPm and o-PD labeling, focusing on whether β3-H267 identifies specific propofol binding site(s). The results show that propofol, but not AziPm protects β3-H267 from labeling by o-PD, whereas both propofol and o-PD protect against AziPm labeling of β3-M286, β3-M227, and α1I239. These data indicate that there are three distinct classes of propofol binding sites, with AziPm binding to two of the classes and o-PD to all three. Analysis of binding stoichiometry using native mass spectrometry in β3 homomeric receptors, demonstrated a minimum of five AziPm labeled residues and three o-PD labeled residues per pentamer, suggesting that there are two distinct propofol binding sites per β-subunit. The native mass spectrometry data, coupled with photolabeling performed in the presence of zinc, indicate that the binding site(s) identified by o-PD are adjacent to, but not within the channel pore, since the pore at the 17' H267 residue can accommodate only one propofol molecule. These data validate the existence of three classes of specific propofol binding sites on α1β3 GABAA receptors.
Keywords: fluorescence resonance energy transfer (FRET); gamma-amino butyric acid (GABA); ligand binding protein; mass spectrometry (MS); neurotransmitter receptor; steroid.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



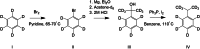
References
-
- Krasowski M.D., Jenkins A., Flood P., Kung A.Y., Hopfinger A.J., Harrison N.L. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. J. Pharmacol. Exp. Ther. 2001;297:338–351. - PubMed
-
- Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. - PubMed
-
- Hales T.G., Lambert J.J. Modulation of the gabaareceptor by propofol. Anesthesiology. 1991;75:A587.
-
- Sanna E., Mascia M.P., Klein R.L., Whiting P.J., Biggio G., Harris R.A. Actions of the general anesthetic propofol on recombinant human GABAA receptors: influence of receptor subunits. J. Pharmacol. Exp. Ther. 1995;274:353–360. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

