Mediators of racial and ethnic inequities in clinical trial participation among patients with cancer, 2011-2023
- PMID: 39271476
- PMCID: PMC11495868
- DOI: 10.1093/jncics/pkae085
Mediators of racial and ethnic inequities in clinical trial participation among patients with cancer, 2011-2023
Abstract
Background: Although racially and ethnically minoritized populations are less likely to participate in cancer trials, it is unknown whether social determinants of health (SDOH) explain these inequities. Here we identify SDOH factors that contribute to racial and ethnic inequities in clinical trial participation among patients with 22 common cancers.
Methods: This retrospective cohort study used electronic health record data (2011-2023) linked to neighborhood (US Census tract) data from multiple sources. Patients were followed from diagnosis to clinical study drug receipt (proxy for trial participation), death, or last recorded activity. Associations were assessed using Cox proportional hazards models adjusted for clinical factors (year of diagnosis, age, sex, performance status, disease stage, cancer type). To elucidate which area-level SDOH underlie racial and ethnic inequities, mediation analysis was performed using nonlinear multiple additive regression tree models.
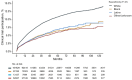
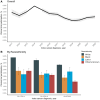
Results: This study included 250 105 patients (64.7% non-Latinx White, 8.9% non-Latinx Black, 5.2% Latinx). Black and Latinx patients were more likely to live in economically or socially marginalized areas (eg, disproportionately minoritized [measure of segregation], limited English proficiency, low vehicle ownership) than White patients. Black (3.7%; hazard ratio = 0.55, 95% confidence interval [CI] = 0.52 to 0.60) and Latinx patients (4.4%; hazard ratio = 0.63, 95% CI = 0.58 to 0.69) were less likely to participate in trials than White patients (6.3%). Fewer patients in economically or socially marginalized neighborhoods participated in trials. Mediators explained 62.2% (95% CI = 49.5% to 74.8%) of participation inequities between Black and White patients; area-level SDOH-including segregation (29.9%, 95% CI = 21.2% to 38.6%) and vehicle ownership (11.6%, 95% CI = 7.0% to 16.1%)-were the most important mediators. Similarly, Latinx-White participation inequities were mediated (65.1%, 95% CI = 49.8% to 80.3%) by area-level SDOH, such as segregation (39.8%, 95% CI = 28.3% to 51.3%), limited English proficiency (11.6%, 95% CI = 2.8% to 20.4%), and vehicle ownership (9.6%, 95% CI = 5.8% to 13.5%).
Conclusions: To improve racial and ethnic diversity in cancer trials, efforts to address barriers related to adverse neighborhood SDOH factors are necessary.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Drs Guadamuz, Wang, Altomare, and Calip report current or previous employment with Flatiron Health, Inc, which is an independent member of the Roche group, and stock ownership in Roche. Dr Guadamuz has current grants from the Robert Wood Johnson Foundation. Dr Calip reports current employment with AbbVie.
Figures
Comment in
-
Structural racism and inequity in cancer clinical trial participation: time for solutions.JNCI Cancer Spectr. 2024 Sep 2;8(5):pkae089. doi: 10.1093/jncics/pkae089. JNCI Cancer Spectr. 2024. PMID: 39438028 Free PMC article. No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
