The DPP-4 inhibitor sitagliptin improves glycaemic control and early-stage diabetic nephropathy in adolescents with type 1 diabetes using the MiniMed 780G advanced hybrid closed-loop system: a randomised controlled trial
- PMID: 39271520
- PMCID: PMC11604790
- DOI: 10.1007/s00125-024-06265-7
The DPP-4 inhibitor sitagliptin improves glycaemic control and early-stage diabetic nephropathy in adolescents with type 1 diabetes using the MiniMed 780G advanced hybrid closed-loop system: a randomised controlled trial
Abstract
Aims/hypothesis: Dipeptidyl peptidase-4 (DPP-4) inhibition has beneficial effects on various metabolic indicators in diabetes. Stromal cell-derived factor-1 (SDF-1) is expressed in diverse organs including the kidneys and is cleaved and inactivated by DPP-4 enzyme. The aim of this study was to conduct a randomised controlled trial to assess the effect of sitagliptin on diabetic nephropathy when used as an add-on therapy to the advanced hybrid closed-loop (AHCL) system in adolescents with type 1 diabetes and nephropathy.
Methods: This open-label, parallel-group, randomised controlled trial took place at the Pediatric Diabetes Clinic, Ain Shams University, Egypt. Forty-six adolescents aged 14.13 ± 2.43 years on the MiniMed 780G system for at least 6 months before study, with HbA1c ≤69 mmol/mol (8.5%) and diabetic nephropathy in the form of microalbuminuria, were randomly assigned to two groups (n=23 for each) based on a computer-generated randomisation sequence. The intervention group received oral sitagliptin 50 mg for 3 months. The other group used AHCL only and served as a control group. The primary outcome measure was the change in urinary albumin/creatinine ratio (UACR) after 3 months of administration of sitagliptin. The key secondary outcome measure was the change from baseline in SDF-1 levels after treatment.
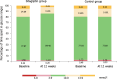
Results: Data for all participants were analysed. No significant difference was found between the groups as regards baseline clinical and laboratory characteristics as well as AHCL system settings (p>0.05). Serum SDF-1 levels were higher in all individuals with type 1 diabetes vs healthy control individuals (p<0.001). After 3 months, sitagliptin resulted in a significant decrease of SDF-1 levels from 3.58 ± 0.73 to 1.99 ± 0.76 ng/ml (p<0.001), together with improvement of UACR from 7.27 ± 2.41 to 1.32 ± 0.31 mg/mmol (p<0.001). In addition, sitagliptin reduced postprandial glucose, sensor glucose, coefficient of variation and total daily dose of insulin, while time in range 3.9-10.0 mmol/l (70-180 mg/dl) and insulin-to-carbohydrate ratio were significantly increased. Sitagliptin was safe and well-tolerated without severe hypoglycaemia or diabetic ketoacidosis.
Conclusions/interpretation: Sitagliptin as an add-on therapy to AHCL had a reno-protective effect for individuals with type 1 diabetes and diabetic nephropathy, in addition to the improvement of time in range while reducing glycaemic variability and without compromising safety.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Trial registration: ClinicalTrials.gov NCT06115460.
Keywords: AHCL glucometrics; Diabetic nephropathy; Fasting lipids; MiniMed 780G; SDF-1; Sitagliptin; Type 1 diabetes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Acknowledgements: The authors thank all the participants and their families for their collaboration during the study. Data availability: The data are available on reasonable request from the authors. Funding: Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Authors’ relationships and activities: The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: All authors (NSE, EAI, MHE-H, MZI, AAE) contributed substantially to the study design, analysis or interpretation of data; critically reviewed, drafted or edited the manuscript; and approved the final work for submission. NSE is responsible for the integrity of the work as a whole.
Figures
References
-
- Yaribeygi H, Sathyapalan T, Sahebkar A (2019) Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sci 234:116776. 10.1016/j.lfs.2019.116776 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

