Diffuse Gastric Cancer: A Comprehensive Review of Molecular Features and Emerging Therapeutics
- PMID: 39271577
- PMCID: PMC11557641
- DOI: 10.1007/s11523-024-01097-2
Diffuse Gastric Cancer: A Comprehensive Review of Molecular Features and Emerging Therapeutics
Abstract
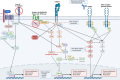
Diffuse-type gastric cancer (DGC) accounts for approximately one-third of gastric cancer diagnoses but is a more clinically aggressive disease with peritoneal metastases and inferior survival compared with intestinal-type gastric cancer (IGC). The understanding of the pathogenesis of DGC has been relatively limited until recently. Multiomic studies, particularly by The Cancer Genome Atlas, have better characterized gastric adenocarcinoma into molecular subtypes. DGC has unique molecular features, including alterations in CDH1, RHOA, and CLDN18-ARHGAP26 fusions. Preclinical models of DGC characterized by these molecular alterations have generated insight into mechanisms of pathogenesis and signaling pathway abnormalities. The currently approved therapies for treatment of gastric cancer generally provide less clinical benefit in patients with DGC. Based on recent phase II/III clinical trials, there is excitement surrounding Claudin 18.2-based and FGFR2b-directed therapies, which capitalize on unique biomarkers that are enriched in the DGC populations. There are numerous therapies targeting Claudin 18.2 and FGFR2b in various stages of preclinical and clinical development. Additionally, there have been preclinical advancements in exploiting unique therapeutic vulnerabilities in several models of DGC through targeting of the focal adhesion kinase (FAK) and Hippo pathways. These preclinical and clinical advancements represent a promising future for the treatment of DGC.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. - PubMed
-
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. 10.1111/apm.1965.64.1.31. - PubMed
-
- Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, et al. Clinicopathological variation of Lauren classification in gastric cancer. Pathol Oncol Res. 2016;22(1):197–202. 10.1007/s12253-015-9996-6. - PubMed
-
- Monster JL, Kemp LJS, Gloerich M, van der Post RS. Diffuse gastric cancer: emerging mechanisms of tumor initiation and progression. Biochim Biophys Acta Rev Cancer. 2022;1877(3): 188719. 10.1016/j.bbcan.2022.188719. - PubMed
-
- WHO Classification of Tumours. Digestive system tumours: WHO classification of tumours, vol. 1. Geneva: World Health Organization; 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

