Synthesis and screening of a library of Lewisx deoxyfluoro-analogues reveals differential recognition by glycan-binding partners
- PMID: 39271664
- PMCID: PMC11399408
- DOI: 10.1038/s41467-024-51081-7
Synthesis and screening of a library of Lewisx deoxyfluoro-analogues reveals differential recognition by glycan-binding partners
Abstract
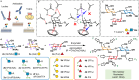
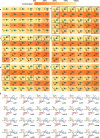
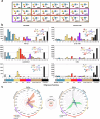
Glycan-mediated interactions play a crucial role in biology and medicine, influencing signalling, immune responses, and disease pathogenesis. However, the use of glycans in biosensing and diagnostics is limited by cross-reactivity, as certain glycan motifs can be recognised by multiple biologically distinct protein receptors. To address this specificity challenge, we report the enzymatic synthesis of a 150-member library of site-specifically fluorinated Lewisx analogues ('glycofluoroforms') using naturally occurring enzymes and fluorinated monosaccharides. Subsequent incorporation of a subset of these glycans into nanoparticles or a microarray revealed a striking spectrum of distinct binding intensities across different proteins that recognise Lewisx. Notably, we show that for two proteins with unique binding sites for Lewisx, glycofluoroforms exhibited enhanced binding to one protein, whilst reduced binding to the other, with selectivity governed by fluorination patterns. We finally showcase the potential diagnostic utility of this approach in glycofluoroform-mediated bacterial toxin detection by lateral flow.
© 2024. The Author(s).
Conflict of interest statement
S.J.R. and M.I.G. are inventors on glycan-diagnostic patent application US 17330986, GB 2007895. The remaining authors declare no competing interests.
Figures


References
-
- Larkin, M. et al. Spectrum of sialylated and nonsialylated fuco-oligosaccharides bound by the endothelial-leukocyte adhesion molecule E-selectin. Dependence of the carbohydrate binding activity on E-selectin density. J. Biol. Chem.267, 13661–13668 (1992). 10.1016/S0021-9258(18)42264-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/M02878X/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M028852/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M028941/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M02847X/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M028976/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M028747/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M028836/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/M011151/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/V50953X/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- WT099197/Z/12/Z/Wellcome Trust (Wellcome)
- 108430/Z/15/Z/Wellcome Trust (Wellcome)
- 218304/Z/19/Z/Wellcome Trust (Wellcome)
- 102576/Z/13/Z/Wellcome Trust (Wellcome)
- ERC-COG: 648239/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- ProgrES-ERC-2017-ADG/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- DTA1799721/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
LinkOut - more resources
Full Text Sources

