A FAPα-activated MRI nanoprobe for precise grading diagnosis of clinical liver fibrosis
- PMID: 39271701
- PMCID: PMC11399433
- DOI: 10.1038/s41467-024-52308-3
A FAPα-activated MRI nanoprobe for precise grading diagnosis of clinical liver fibrosis
Abstract
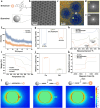
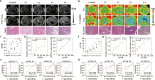
Molecular imaging holds the potential for noninvasive and accurate grading of liver fibrosis. It is limited by the lack of biomarkers that strongly correlate with liver fibrosis grade. Here, we discover the grading potential of fibroblast activation protein alpha (FAPα) for liver fibrosis through transcriptional analysis and biological assays on clinical liver samples. The protein and mRNA expression of FAPα are linearly correlated with fibrosis grade (R2 = 0.89 and 0.91, respectively). A FAPα-responsive MRI molecular nanoprobe is prepared for quantitatively grading liver fibrosis. The nanoprobe is composed of superparamagnetic amorphous iron nanoparticles (AFeNPs) and paramagnetic gadoteric acid (Gd-DOTA) connected by FAPα-responsive peptide chains (ASGPAGPA). As liver fibrosis worsens, the increased FAPα cut off more ASGPAGPA, restoring a higher T1-MRI signal of Gd-DOTA. Otherwise, the signal remains quenched due to the distance-dependent magnetic resonance tuning (MRET) effect between AFeNPs and Gd-DOTA. The nanoprobe identifies F1, F2, F3, and F4 fibrosis, with area under the curve of 99.8%, 66.7%, 70.4%, and 96.3% in patients' samples, respectively. This strategy exhibits potential in utilizing molecular imaging for the early detection and grading of liver fibrosis in the clinic.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- SRA/PRJNA1137073
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

