Incorporation of nitrogen in antinutritional Solanum alkaloid biosynthesis
- PMID: 39271954
- PMCID: PMC11666457
- DOI: 10.1038/s41589-024-01735-w
Incorporation of nitrogen in antinutritional Solanum alkaloid biosynthesis
Abstract
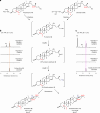
Steroidal glycoalkaloids (SGAs) are specialized metabolites produced by hundreds of Solanum species including food crops, such as tomato, potato and eggplant. Unlike true alkaloids, nitrogen is introduced at a late stage of SGA biosynthesis through an unknown transamination reaction. Here, we reveal the mechanism by which GLYCOALKALOID METABOLISM12 (GAME12) directs the biosynthesis of nitrogen-containing steroidal alkaloid aglycone in Solanum. We report that GAME12, a neofunctionalized γ-aminobutyric acid (GABA) transaminase, undergoes changes in both active site specificity and subcellular localization to switch from its renown and generic activity in core metabolism to function in a specialized metabolic pathway. Moreover, overexpression of GAME12 alone in engineered S. nigrum leaves is sufficient for de novo production of nitrogen-containing SGAs. Our results highlight how hijacking a core metabolism GABA shunt enzyme is crucial in numerous Solanum species for incorporating a nitrogen to a steroidal-specialized metabolite backbone and form defensive alkaloids.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Steroidal scaffold decorations in Solanum alkaloid biosynthesis.Mol Plant. 2024 Aug 5;17(8):1236-1254. doi: 10.1016/j.molp.2024.06.013. Epub 2024 Jun 26. Mol Plant. 2024. PMID: 38937971
-
Characterization of C-26 aminotransferase, indispensable for steroidal glycoalkaloid biosynthesis.Plant J. 2021 Oct;108(1):81-92. doi: 10.1111/tpj.15426. Epub 2021 Aug 9. Plant J. 2021. PMID: 34273198
-
Short-chain dehydrogenase/reductase governs steroidal specialized metabolites structural diversity and toxicity in the genus Solanum.Proc Natl Acad Sci U S A. 2018 Jun 5;115(23):E5419-E5428. doi: 10.1073/pnas.1804835115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784829 Free PMC article.
-
The Therapeutic Value of Solanum Steroidal (Glyco)Alkaloids: A 10-Year Comprehensive Review.Molecules. 2023 Jun 23;28(13):4957. doi: 10.3390/molecules28134957. Molecules. 2023. PMID: 37446619 Free PMC article. Review.
-
The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism.Phytochemistry. 2015 May;113:24-32. doi: 10.1016/j.phytochem.2014.12.010. Epub 2014 Dec 31. Phytochemistry. 2015. PMID: 25556315 Review.
Cited by
-
Identification of genes involved in verazine biosynthesis in Veratrum grandiflorum and their heterologous production in Saccharomyces cerevisiae.BMC Plant Biol. 2025 Jul 3;25(1):853. doi: 10.1186/s12870-025-06899-8. BMC Plant Biol. 2025. PMID: 40610854 Free PMC article.
-
Alkaloid production of Solanum elaeagnifolium Cav from callus for anticancer potential using gene expression of cancer-related genes.PLoS One. 2025 Aug 22;20(8):e0329977. doi: 10.1371/journal.pone.0329977. eCollection 2025. PLoS One. 2025. PMID: 40845071 Free PMC article.
-
Physiological and Molecular Mechanisms of Light-Induced Greening in Potatoes: A Path to Food Safety.Foods. 2025 May 19;14(10):1798. doi: 10.3390/foods14101798. Foods. 2025. PMID: 40428577 Free PMC article. Review.
-
Synthetic Biology in Natural Product Biosynthesis.Chem Rev. 2025 Apr 9;125(7):3814-3931. doi: 10.1021/acs.chemrev.4c00567. Epub 2025 Mar 21. Chem Rev. 2025. PMID: 40116601 Review.
-
A million shades of green: understanding and harnessing plant metabolic diversity.EMBO J. 2025 Aug;44(16):4409-4418. doi: 10.1038/s44318-025-00496-z. Epub 2025 Jul 3. EMBO J. 2025. PMID: 40610793 Free PMC article. Review.
References
-
- Schreiber, K. in The Alkaloids: Chemistry and Physiology (ed. Manske, R. H. F.) (Academic Press, 1968).
-
- Roddick, J. G. in Saponins Used in Traditional and Modern Medicine (eds Waller, G. R. & Yamasaki, K.) (Springer, 1996).
-
- Lichman, B. R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep.38, 103–129 (2021). - PubMed
-
- Itkin, M. et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science341, 175–179 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

