Clinical and radiological pattern of olaparib-induced interstitial lung disease
- PMID: 39272066
- PMCID: PMC11396475
- DOI: 10.1186/s12890-024-03276-3
Clinical and radiological pattern of olaparib-induced interstitial lung disease
Abstract
Background: PARP inhibitors (PARPi) are used in the treatment of ovarian, breast, pancreatic, and prostate cancers. Pneumonitis has been identified as a potential side effect, with a higher meta-analysis-assessed risk for olaparib versus other PARPi. Olaparib-induced interstitial lung disease (O-ILD) was first described within the Japanese population, with few information available for Caucasian patients.
Methods: We performed a retrospective study by pooling data from the French and Belgian pharmacovigilance databases from 2018 to 2022. Patients with O-ILD were included following a central review by: 1) pharmacologists using the French drug causality assessment method; 2) senior pneumologists or radiologists, using the Fleischner Society's recommendations.
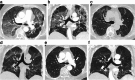
Results: Five patients were identified and analysed. All were females, with ovarian or breast cancer. Median age at O-ILD diagnosis was 71 (38-72) years old, with no smoking history. Median delay between treatment initiation and symptom occurrence was 12 (6-33) weeks. Pneumonitis severity assessed using the Common Terminology Criteria for Adverse Events V5 was Grade 3 (n = 4) or 2 (n = 1). CT-scan review (n = 3) described hypersensitivity pneumonitis reaction as a common pattern. Bronchioalveolar lavage (n = 4) revealed lymphocytic alveolitis. Treatments relied on olaparib discontinuation (n = 5) and glucocorticoid intake (n = 4), with no fatal issue. Safe re-challenge with PARPi occurred in two patients. Forty additional O-ILD cases were identified in the WHO VigiBase database, including one fatal case.
Conclusions: PARPi-ILD is a rare but potentially life-threatening disease, presenting as a hypersensitivity pneumonitis pattern within 3 months of PARPi initiation. Treatment primarily relies on medication discontinuation. Re-challenging with another PARPi could be considered.
Clinical trial number: CEPRO #2023-010.
Keywords: Adverse effect; Hypersensibility pneumonitis; Interstitial Lung Disease; PARPi.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N, Mäenpää J, Selle F, Sehouli J, Lorusso D, Guerra Alía EM, Reinthaller A, Nagao S, Lefeuvre-Plesse C, Canzler U, Scambia G, Lortholary A, Marmé F, Combe P, de Gregorio N, Rodrigues M, Buderath P, Dubot C, Burges A, You B, Pujade-Lauraine E, Harter P, PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–28. 10.1056/NEJMoa1911361 - DOI - PubMed
-
- Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park J-O, Hochhauser D, Arnold D, Oh D-Y, Reinacher-Schick A, Tortora G, Algül H, O’Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381(4):317–27. 10.1056/NEJMoa1903387 - DOI - PMC - PubMed
-
- de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–102. 10.1056/NEJMoa1911440 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

