Quality assessment of common anti-malarial medicines marketed in Gambella, National Regional State, South Western-Ethiopia
- PMID: 39272079
- PMCID: PMC11401441
- DOI: 10.1186/s12936-024-05091-x
Quality assessment of common anti-malarial medicines marketed in Gambella, National Regional State, South Western-Ethiopia
Abstract
Background: Over the past years, there has been a growing concern that a considerable amount of anti-malarial supply in the underdeveloped world particularly in the private sector, is of poor quality. The World Health Organization (WHO) has received about 1500 reports that mentions instances of substandard and falsified products since 2013. The majority of the reports concerned antibiotics and anti-malarials. The majority of reports (42%) originate from the WHO African region.
Objective: This study intends to assess the quality of the most widely used anti-malarial medications [artemether-lumefantrine tablets, chloroquine phosphate tablets, primaquine phosphate tablets, artesunate, and artemether injections] in Gambella, South-West, Ethiopia.
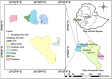
Methods: A total of 52 samples were collected on June 2022 from Gambella National Regional State, Ethiopia. Half of the districts (six) located in the four zones of the region were chosen using simple random sampling technique. All drug retail outlets available in the selected districts (locally known as woredas) were included. The samples were subjected to visual inspection with a tool adopted from the joint WHO/FIP/ USP checklist. The pharmacopeial tests for identification, uniformity of dosage forms, assay, thickness, diameter, hardness, friability, disintegration test, dissolution, and sterility tests were carried out according to the USP 44-NF 39 and International Pharmacopoeia 11th edition, 2022 monographs.
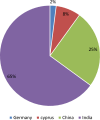
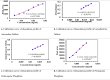
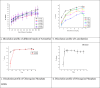
Results and discussion: Only 25% of the samples were registered on the Ethiopian Food and Drug Authority (EFDA's) electronic regulatory/ registration system (ERIS). Besides, 88.8% of artemether injection products were presented in clear glass ampoules. This might expose the products to photochemical degradation that leads to in loss of anti-plasmodial activity. In addition, 50% of the artemether products assessed were not bioequivalent with the comparator product in the in vitro dissolution comparison tests. Overall, the study findings reveal a high prevalence (58.3%) of substandard anti-malarial drugs in the region. The stated percent of the samples had failed in one or more of the quality test parameters assessed in this study.
Conclusion: The study findings reveal a high prevalence (58.3%) of substandard anti-malarial drugs in the region. Only a quarter were registered and 38% of the unregistered products failed the quality tests. Hence, the national, regional medicine regulatory bodies and other stake holders should perform the required roles to circumvent presence of Substandard and Falsified (SF) anti-malarial drugs in the study sites.
Keywords: Antimalarials; Ethiopia; Gambella; Malaria; Poor quality; Quality assessment; Substandard and falsified (SF) medicines.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Dembele O. Risk-based post-marketing surveillance (RB-PMS2) of antimalarial and MNCH drugs in Mali (PY2). Int J Pharm Bio-Med Sci. 2022;2:09. 10.47191/ijpbms/v2-i9-06 - DOI
-
- WHO. World malaria report 2020: 20 years of global progress and challenges. [Internet]. Geneva, World Health Organization, 2020. https://apps.who.int/iris/handle/10665/337660. (Accessed: 6 September 2022).
-
- WHO. World malaria report 2022. . [Internet]. Geneva, World Health Organization, 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria.... [Accessed 29 May 2023].
-
- Al-Worafi YM. Counterfeit and substandard medications drug safety in `developing countries: achievements and challenges. London: Elsevier; 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous