Repeated COVID-19 mRNA vaccination results in IgG4 class switching and decreased NK cell activation by S1-specific antibodies in older adults
- PMID: 39272189
- PMCID: PMC11401348
- DOI: 10.1186/s12979-024-00466-9
Repeated COVID-19 mRNA vaccination results in IgG4 class switching and decreased NK cell activation by S1-specific antibodies in older adults
Abstract
Background: Previous research has shown that repeated COVID-19 mRNA vaccination leads to a marked increase of SARS-CoV-2 spike-specific serum antibodies of the IgG4 subclass, indicating far-reaching immunoglobulin class switching after booster immunization. Considering that repeated vaccination has been recommended especially for older adults, the aim of this study was to investigate IgG subclass responses in the ageing population and assess their relation with Fc-mediated antibody effector functionality.
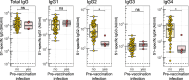
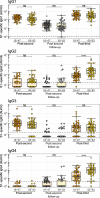
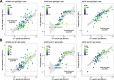
Results: Spike S1-specific IgG subclass concentrations (expressed in arbitrary units per mL), antibody-dependent NK cell activation, complement deposition and monocyte phagocytosis were quantified in serum from older adults (n = 38-50, 65-83 years) at one month post-second, -third and -fifth vaccination. Subclass distribution in serum was compared to that in younger adults (n = 64, 18-47 years) at one month post-second and -third vaccination. Compared to younger individuals, older adults showed increased levels of IgG2 and IgG4 at one month post-third vaccination (possibly related to factors other than age) and a further increase following a fifth dose. The capacity of specific serum antibodies to mediate NK cell activation and complement deposition relative to S1-specific total IgG concentrations decreased upon repeated vaccination. This decrease associated with an increased IgG4/IgG1 ratio.
Conclusions: In conclusion, these findings show that, like younger individuals, older adults produce antibodies with reduced functional capacity upon repeated COVID-19 mRNA vaccination. Additional research is needed to better understand the mechanisms underlying these responses and their potential implications for vaccine effectiveness. Such knowledge is vital for the future design of optimal vaccination strategies in the ageing population.
Keywords: Ageing; Booster; Complement; Fc-effector functions; IgG subclasses.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- European Centre for Disease Prevention and Control. Interim public health considerations for COVID-19 vaccination roll-out during 2023. Stockholm: ECDC; 2023 5 April 2023.
-
- Ravussin A, Robertson AH, Wolf AS, Blix K, Kjonstad IF, Solum G, et al. Determinants of humoral and cellular immune responses to three doses of mRNA SARS-CoV-2 vaccines in older adults: a longitudinal cohort study. Lancet Healthy Longev. 2023;4(5):e188–99. 10.1016/S2666-7568(23)00055-7 - DOI - PMC - PubMed
-
- Tan NH, Geers D, Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, et al. Immunogenicity of bivalent omicron (BA.1) booster vaccination after different priming regimens in health-care workers in the Netherlands (SWITCH ON): results from the direct boost group of an open-label, multicentre, randomised controlled trial. Lancet Infect Dis. 2023;23(8):901–13. 10.1016/S1473-3099(23)00140-8 - DOI - PMC - PubMed
-
- Munro APS, Feng S, Janani L, Cornelius V, Aley PK, Babbage G, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22(8):1131–41. 10.1016/S1473-3099(22)00271-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

