The BCL11A transcription factor stimulates the enzymatic activities of the OGG1 DNA glycosylase
- PMID: 39272221
- PMCID: PMC11712033
- DOI: 10.1515/hsz-2024-0088
The BCL11A transcription factor stimulates the enzymatic activities of the OGG1 DNA glycosylase
Abstract
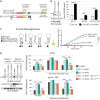
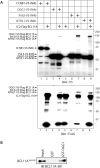
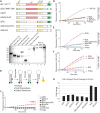
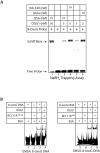
The BCL11A transcription factor has previously been shown to interact with and stimulate the enzymatic activities of the NTHL1 DNA glycosylase and Pol β polymerase. Here we show that BCL11A and a smaller peptide encompassing amino acids 160 to 520 can interact with the 8-oxoguanine DNA glycosylase, OGG1, increase the binding of OGG1 to DNA that contains an 8-oxoguanine base and stimulate the glycosylase activity of OGG1. Following BCL11A knockdown, we observed an increase in oxidized purines in the genome using comet assays, while immunoassays reveal an increase in 8-oxoG bases. Structure-function analysis indicates that the stimulation of OGG1 by BCL11A requires the zinc fingers 1, 2 and 3 as well as the proline-rich region between the first and second zing finger, but a glutamate-rich region downstream of zinc finger 3 is dispensable. Ectopic expression of a small peptide that contains the three zinc fingers can rescue the increase in 8-oxoguanine caused by BCL11A knockdown. These findings, together with previous results showing that BCL11A stimulates the enzymatic activities of NTHL1 and the Pol β polymerase, suggest that high expression of BCL11A is important to protect cancer cells against oxidative DNA damage.
Keywords: 8-oxoguanine glycosylase (OGG1); BCL11A; base excision repair; breast cancer; reactive oxygen species (ROS); transcription factor.
© 2024 Walter de Gruyter GmbH, Berlin/Boston.
Conflict of interest statement
Competing interests: The authors state no conflict of interest.
Figures


Similar articles
-
OGG1 augments the transcriptional activation of Foxp3 to promote iTreg differentiation for IBD alleviation.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2424733122. doi: 10.1073/pnas.2424733122. Epub 2025 Jul 22. Proc Natl Acad Sci U S A. 2025. PMID: 40694333 Free PMC article.
-
Capturing a glycosylase reaction intermediate in DNA repair by freeze-trapping of a pH-responsive hOGG1 mutant.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf718. doi: 10.1093/nar/gkaf718. Nucleic Acids Res. 2025. PMID: 40754315 Free PMC article.
-
Solution structure of the Z0 domain from transcription repressor BCL11A sheds light on the sequence properties of protein-binding zinc fingers.Protein Sci. 2025 Apr;34(4):e70097. doi: 10.1002/pro.70097. Protein Sci. 2025. PMID: 40099876
-
Impact of DNA polymorphisms in key DNA base excision repair proteins on cancer risk.Hum Exp Toxicol. 2012 Oct;31(10):981-1005. doi: 10.1177/0960327112444476. Epub 2012 Sep 27. Hum Exp Toxicol. 2012. PMID: 23023028 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Avram D., Fields A., Pretty On Top K., Nevrivy D.J., Ishmael J.E., Leid M. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J. Biol. Chem. . 2000;275:10315–10322. doi: 10.1074/jbc.275.14.10315. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
