The Impact of Liquid Biopsy in Advanced Ovarian Cancer Care
- PMID: 39272653
- PMCID: PMC11394565
- DOI: 10.3390/diagnostics14171868
The Impact of Liquid Biopsy in Advanced Ovarian Cancer Care
Abstract
Introduction: Ovarian cancer is the third most common gynaecological cancer and has a very high mortality rate. The cornerstone of treatment is complete debulking surgery plus chemotherapy. Even with treatment, 80% of patients have a recurrence. Circulating tumour DNA (ctDNA) has been shown to be useful in the control and follow-up of some tumours. It could be an option to define complete cytoreduction and for the early diagnosis of recurrence.
Objective: We aimed to demonstrate the usefulness of ctDNA and cell-free DNA (cfDNA) as a marker of complete cytoreduction and during follow-up in patients with advanced ovarian cancer.
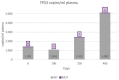
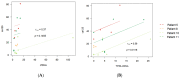
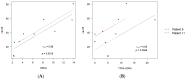
Material and methods: We selected 22 women diagnosed with advanced high-grade serous ovarian cancer, of which only 4 had complete records. We detected cfDNA by polymerase chain reaction (PCR), presented as ng/mL, and detected ctDNA with droplet digital PCR (ddPCR). We calculated Pearson correlation coefficients to evaluate correlations among cfDNA, ctDNA, and cancer antigen 125 (CA125), a biomarker.
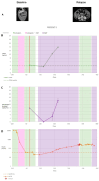
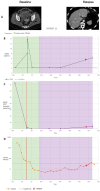
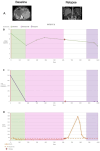
Results: The results obtained in the evaluation of cfDNA and ctDNA and their correlation with tumour markers and the radiology of patients with complete follow-up show disease progression during the disease, stable disease, or signs of recurrence. cfDNA and ctDNA correlated significantly with CA125. Following cfDNA and ctDNA over time indicated a recurrence several months earlier than computed tomography and CA125 changes.
Conclusion: An analysis of cfDNA and ctDNA offers a non-invasive clinical tool for monitoring the primary tumour to establish a complete cytoreduction and to diagnose recurrence early.
Keywords: advanced ovarian cancer; cancer care; liquid biopsy; translational research.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Winter W.E., III, Maxwell G.L., Tian C., Carlson J.W., Ozols R.F., Rose P.G., Markman M., Armstrong D.K., Muggia F., McGuire W.P. Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

