Circulating Multiple Myeloma Cells (CMMCs) as Prognostic and Predictive Markers in Multiple Myeloma and Smouldering MM Patients
- PMID: 39272787
- PMCID: PMC11393854
- DOI: 10.3390/cancers16172929
Circulating Multiple Myeloma Cells (CMMCs) as Prognostic and Predictive Markers in Multiple Myeloma and Smouldering MM Patients
Abstract
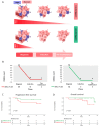
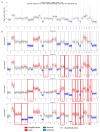
In recent years, liquid biopsy has emerged as a promising alternative to the bone marrow (BM) examination, since it is a minimally invasive technique allowing serial monitoring. Circulating multiple myeloma cells (CMMCs) enumerated using CELLSEARCH® were correlated with patients' prognosis and measured under treatment to assess their role in monitoring disease dynamics. Forty-four MM and seven smouldering MM (SMM) patients were studied. The CMMC medians at diagnosis were 349 (1 to 39,940) and 327 (range 22-2463) for MM and SMM, respectively. In the MM patients, the CMMC count was correlated with serum albumin, calcium, β2-microglobulin, and monoclonal components (p < 0.04). Under therapy, the CMMCs were consistently detectable in 15/40 patients (coMMstant = 1) and were undetectable or decreasing in 25/40 patients (coMMstant = 0). High-quality response rates were lower in the coMMstant = 1 group (p = 0.04), with a 7.8-fold higher risk of death (p = 0.039), suggesting that continuous CMMC release is correlated with poor responses. In four MM patients, a single-cell DNA sequencing analysis on residual CMMCs confirmed the genomic pattern of the aberrations observed in the BM samples, also highlighting the presence of emerging clones. The CMMC kinetics during treatment were used to separate the patients into two subgroups based on the coMMstant index, with different responses and survival probabilities, providing evidence that CMMC persistence is associated with a poor disease course.
Keywords: circulating tumor cells; liquid biopsy; multiple myeloma; single-cell analysis; smouldering multiple myeloma.
Conflict of interest statement
M.T., P.T., M.G., A.F., and N.M. are employees of Menarini Silicon Biosystems SpA, manufacturer of CELLSEARCH, DEPArray and Ampli1; P.T., M.G., A.F., and N.M. are co-inventors (not receiving royalties) on various patents assigned to Menarini Silicon Biosystems, related to the aforementioned technologies.
Figures



References
-
- An G., Qin X., Acharya C., Xu Y., Deng S., Shi L., Zang M., Sui W., Yi S., Li Z., et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann. Hematol. 2015;94:257–264. doi: 10.1007/s00277-014-2211-0. - DOI - PubMed
-
- Palumbo A., Avet-Loiseau H., Oliva S., Lokhorst H.M., Goldschmidt H., Rosinol L., Richardson P., Caltagirone S., Lahuerta J.J., Facon T., et al. Revised International Staging System for Multiple Myeloma: A Report from International Myeloma Working Group. J. Clin. Oncol. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. - DOI - PMC - PubMed
-
- Gonsalves W.I., Jevremovic D., Nandakumar B., Dispenzieri A., Buadi F.K., Dingli D., Lacy M.Q., Hayman S.R., Kapoor P., Leung N., et al. Enhancing the R-ISS classification of newly diagnosed multiple myeloma by quantifying circulating clonal plasma cells. Am. J. Hematol. 2020;95:310–315. doi: 10.1002/ajh.25709. - DOI - PMC - PubMed
-
- Garcés J.J., Cedena M.T., Puig N., Burgos L., Perez J.J., Cordon L., Flores-Montero J., Sanoja-Flores L., Calasanz M.J., Ortiol A., et al. Circulating Tumor Cells for the Staging of Patients with Newly Diagnosed Transplant-Eligible Multiple Myeloma. J. Clin. Oncol. 2022;40:3151–3161. doi: 10.1200/JCO.21.01365. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

