Sex Difference in Disease-Related Adverse Events Post-Diagnosis of Lung Cancer Brain Metastases in Medicare Individuals ≥ 66 Years of Age
- PMID: 39272844
- PMCID: PMC11394199
- DOI: 10.3390/cancers16172986
Sex Difference in Disease-Related Adverse Events Post-Diagnosis of Lung Cancer Brain Metastases in Medicare Individuals ≥ 66 Years of Age
Abstract
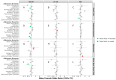
Sex differences are evident in adverse events (AEs) related to brain tumors, yet sex differences in AEs specific to brain metastases (BrMs) are underexplored. Lung cancer BrMs dominate among BrM, comprising over half of cases. This study examined sex differences in AEs associated with lung cancer BrMs in individuals aged 66 or older using the SEER-Medicare dataset. Multivariable logistic regression, adjusted for demographic factors and comorbidities, stratified by histological subtype, treatment, age, and year of diagnosis were used to analyze AEs among those with BrMs from primary lung tumors. Year of diagnosis was grouped into prior/post-2013, to account for shifts in treatment paradigms. The results showed nuanced sex-specific AEs. Females diagnosed post-2013 with small-cell, squamous-cell, or other non-small-cell carcinoma BrMs had a higher headache likelihood than males. Males with adenocarcinoma post-2013 were more likely to experience brain herniation. Females aged 76 and older with small-cell BrM exhibited increased vision difficulty risk compared to males of the same age, with no significant difference in other age groups. Males treated for adenocarcinoma faced heightened hemorrhagic stroke risk. This study reveals sex-specific disparities in AEs among older individuals with lung cancer BrMs, varying by histological subtype, age, diagnosis year, and treatment.
Keywords: adverse events; brain metastases; lung cancer; sex differences.
Conflict of interest statement
The authors have no conflicts of interest to declare. Jill S. Barnholtz-Sloan is a full-time paid employee of the NIH/NCI. Mantas Dmukauskas is a full-time fellow of the NIH/NCI. Gino Cioffi and Kristin A. Waite are full-time contractors of the NIH/NCI. Patrick C. Ma has received a consulting honorarium from AstraZeneca and BeiGene.
Figures




References
-
- Zhang Y., Vaccarella S., Morgan E., Li M., Etxeberria J., Chokunonga E., Manraj S.S., Kamate B., Omonisi A., Bray F. Global variations in lung cancer incidence by histological subtype in 2020: A population-based study. Lancet Oncol. 2023;24:1206–1218. doi: 10.1016/S1470-2045(23)00444-8. - DOI - PubMed
-
- Cagney D.N., Martin A.M., Catalano P.J., Redig A.J., Lin N.U., Lee E.Q., Wen P.Y., Dunn I.F., Bi W.L., Weiss S.E., et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 2017;19:1511–1521. doi: 10.1093/neuonc/nox077. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

