Significance of P53-Binding Protein 1 as a Novel Molecular Histological Marker for Hypopharyngeal Squamous Neoplasms
- PMID: 39272845
- PMCID: PMC11394016
- DOI: 10.3390/cancers16172987
Significance of P53-Binding Protein 1 as a Novel Molecular Histological Marker for Hypopharyngeal Squamous Neoplasms
Abstract
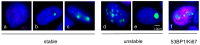
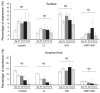
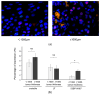
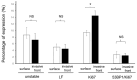
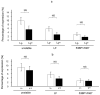
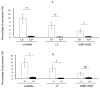
The DNA damage response protein p53-binding protein 1 (53BP1) accumulates and forms foci at double-strand DNA breaks, indicating the extent of DNA instability. However, the potential role of 53BP1 as a molecular biomarker for hypopharyngeal squamous cell carcinoma (HPSCC) diagnosis remains unknown. Here, we evaluated the potential of immunofluorescence-based analysis of 53BP1 expression to differentiate the histology of hypopharyngeal neoplasms. A total of 125 lesions from 39 surgically or endoscopically resected specimens from patients with HPSCC was histologically evaluated. 53BP1 expression in the nucleus was examined using immunofluorescence. The number of 53BP1 nuclear foci increased with the progression from non-tumorous to low-grade dysplasia, high-grade dysplasia, and squamous cell carcinoma. Unstable 53BP1 expression served as an independent factor for distinguishing lesions that required intervention. Colocalization of 53BP1 foci in proliferating cells, as assessed by Ki67, was increased in tumors ≥ 1000 µm in depth compared to those <1000 µm in depth at the tumor surface. Hence, the expression patterns of nuclear 53BP1 foci were associated with the progression of hypopharyngeal neoplasms. These findings suggest that 53BP1 could serve as an ancillary marker to support histological diagnosis and predict the factors that influence prognosis in patients with HPSCC.
Keywords: DNA double-strand break; genomic instability; hypopharyngeal squamous cell carcinoma; p53-binding protein 1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kim S.-Y., Rho Y.-S., Choi E.-C., Kim M.-S., Woo J.-H., Lee D.H., Chung E.J., Park M.W., Kim D.-H., Joo Y.-H. Clinicopathological Factors Influencing the Outcomes of Surgical Treatment in Patients with T4a Hypopharyngeal Cancer. BMC Cancer. 2017;17:904. doi: 10.1186/s12885-017-3880-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

