Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Gastric Cancer Peritoneal Metastases: Results from the Lithuanian PIPAC Program
- PMID: 39272850
- PMCID: PMC11394265
- DOI: 10.3390/cancers16172992
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Gastric Cancer Peritoneal Metastases: Results from the Lithuanian PIPAC Program
Abstract
Background: Peritoneal metastases (PM) of gastric cancer (GC) are considered a terminal condition, with reported median survival ranging from 2 to 9 months. Standard treatment typically involves systemic chemotherapy alone or combined with targeted therapy or immunotherapy, though efficacy is limited. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) has emerged as a novel technique for treating GC PM, although it remains an experimental treatment under investigation. This study aimed to summarize the outcomes of GC PM treatment with PIPAC from the Lithuanian PIPAC program.
Methods: All patients who underwent PIPAC for GC PM at Vilnius University Hospital Santaros Klinikos between 2015 and 2022 were included in this retrospective study. The safety of PIPAC was assessed by postoperative complications according to the Clavien-Dindo classification. Efficacy was evaluated based on the peritoneal carcinomatosis index (PCI), ascites dynamics throughout the treatment, and long-term outcomes.
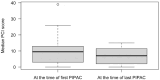
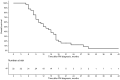
Results: In total, 32 patients underwent 71 PIPAC procedures. Intraoperative and postoperative morbidity related to PIPAC occurred after three (4.2%) procedures. Following PIPAC, there was a tendency towards a decrease in median PCI from 10 (Q1 3; Q3 13) to 7 (Q1 2; Q3 12), p = 0.75, and a decrease in median ascites volume from 1300 mL (Q1 500; Q3 3600) at the first PIPAC to 700 mL (Q1 250; Q3 4750) at the last PIPAC, p = 0.56; however, these differences were not statistically significant. The median overall survival after PM diagnosis was 12.5 months (95% CI 10-17), and the median survival after the first PIPAC procedure was 5 months (95% CI 4-10).
Conclusions: PIPAC is a safe and feasible treatment option for GC PM; however, well-designed prospective studies are needed to fully assess its efficacy.
Keywords: gastric cancer; peritoneal metastasis; pipac.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this study. This research did not receive any external funding. Some of the results presented in this manuscript have been shared with the scientific community at various national and international conferences.
Figures

References
-
- Ellebæk S.B., Graversen M., Detlefsen S., Lundell L., Fristrup C.W., Pfeiffer P., Mortensen M.B. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) of Peritoneal Metastasis from Gastric Cancer: A Descriptive Cohort Study. Clin. Exp. Metastasis. 2020;37:325–332. doi: 10.1007/s10585-020-10023-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

