Accuracy of GynTect® Methylation Markers to Detect Recurrent Disease in Patients Treated for CIN3: A Proof-of-Concept Case-Control Study
- PMID: 39272880
- PMCID: PMC11394525
- DOI: 10.3390/cancers16173022
Accuracy of GynTect® Methylation Markers to Detect Recurrent Disease in Patients Treated for CIN3: A Proof-of-Concept Case-Control Study
Abstract
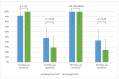
Post-treatment follow-up in women with CIN3 is mandatory due to relapse in up to 15% of patients within 2 years. Standard follow-up care based on hrHPV-DNA/cytology co-testing has high sensitivity but limited specificity. The aim of our proof-of-concept case-control study was to evaluate the performance of the methylation test GynTect® for the detection of recurrent CIN2/3 during follow-up. Residual clinical material from a recent, prospective, multicenter, observational study was available for further analysis. We studied a sample of 17 cases with recurrent CIN2/3 diagnosed within 24 months of follow-up and 31 controls without recurrence. DNA from cervical scrapes at baseline (immediately before CIN3 surgery) and up to three follow-up visits were analyzed for hrHPV and GynTect® methylation status. Cytology data were available from the previous study. Overall, 12 cases and 21 controls were GynTect-positive at baseline. In these subgroups, single test sensitivity at first follow-up was 67% (95% CI 39-87%) for GynTect® compared to 83% (95% CI 55-96%) for hrHPV (p = 0.50). Single test specificity was significantly higher for GynTect® (90%, 95% CI 71-98% vs. 62%, 95% CI 40-80%) (p = 0.03). In a co-testing setting, both hrHPV/cytology and GynTect®/cytology detected all recurrences. Specificity for GynTect®/cytology was higher than for hrHPV/cytology, but this difference was not statistically significant. In conclusion, for initially GynTect-positive patients, both hrHPV and GynTect® tests detected recurrent disease with similar sensitivity, but the GynTect® assay has a higher specificity. Incident hrHPV infection and/or persisting multifocal hrHPV infections without clinical disease are most likely responsible for the poorer specificity of the hrHPV test. A future prospective validation study will have to show whether GynTect®/cytology co-testing can outperform hrHPV/cytology co-testing in post-treatment surveillance.
Keywords: DNA methylation; cervical intraepithelial neoplasia (CIN); hrHPV; post-treatment surveillance; recurrent CIN.
Conflict of interest statement
KW and MS are employees of oncgnostics GmbH. MS and MD are minority shareholders of oncgnostics GmbH. The employees of oncgnostics had no influence on the study design, sample collection, interpretation of data and were not involved in drafting this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Schneider A., Hoyer H., Lotz B., Leistritza S., Kühne-Heid R., Nindl I., Müller B., Haerting J., Dürst M. Screening for high-grade cervical intra-epithelial neoplasia and cancer by testing for high-risk HPV, routine cytology or colposcopy. Int. J. Cancer. 2000;89:529–534. doi: 10.1002/1097-0215(20001120)89:6<529::AID-IJC11>3.0.CO;2-G. - DOI - PubMed
-
- Bulkmans N.W., Rozendaal L., Snijders P.J., Voorhorst F.J., Boeke A.J., Zandwijken G.R., van Kemenade F.J., Verheijen R.H., v Groningen K., Boon M.E., et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: Design, Methods and Baseline Data of 44,102 Women. Int. J. Cancer. 2004;110:94–101. doi: 10.1002/ijc.20076. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources