Therapeutical Usefulness of PD-1/PD-L1 Inhibitors in Aggressive or Metastatic Pituitary Tumours
- PMID: 39272895
- PMCID: PMC11394371
- DOI: 10.3390/cancers16173033
Therapeutical Usefulness of PD-1/PD-L1 Inhibitors in Aggressive or Metastatic Pituitary Tumours
Abstract
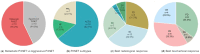
Therapeutic options for pituitary neuroendocrine tumours (PitNETs) refractory to temozolomide are scarce. Immune checkpoint inhibitors (ICIs), particularly inhibitors of the programmed cell death-1 (PD-1) pathway and its ligand (PD-L1), have been experimentally used in aggressive or metastatic PitNETs. We aimed to study the therapeutic usefulness of anti-PD-1 drugs in patients with aggressive or metastatic PitNETs. Published cases and case series involving patients with PitNETs treated with PD-1/PD-L1 inhibitors were reviewed. Demographic data, clinical-pathological features, previous therapies, drug dosage and posology, and the best radiological and biochemical responses, as well as survival data, were evaluated. We identified 29 cases of aggressive (n = 13) or metastatic (n = 16) PitNETs treated with PD-1/PD-L1 inhibitors. The hypersecretion of adrenocorticotropic hormone (ACTH) was documented in eighteen cases (62.1%), seven were prolactinomas (24.1%), and four were non-functioning PitNETs. All patients underwent various therapies prior to using ICIs. Overall, a positive radiological response (i.e., partial/complete radiological response and stable disease) was observed in eighteen of twenty-nine cases (62.1%), of which ten and four were ACTH- and prolactin-secreting PitNETs, respectively. Hormonal levels reduced or stabilised after using ICIs in 11 of the 17 functioning PitNET cases with available data (64.7%). The median survival of patients treated with ICIs was 13 months, with a maximum of 42 months in two ACTH-secreting tumours. Among 29 patients with PitNETs treated with PD-1/PD-L1 inhibitors, the positive radiological and biochemical response rates were 62.1% and 64.7%, respectively. Altogether, these data suggest a promising role of ICIs in patients with aggressive or metastatic PitNETs refractory to other treatment modalities.
Keywords: PD-1; PD-L1; immune checkpoint inhibitors; immunotherapy; ipilimumab; nivolumab; pembrolizumab; pituitary adenoma; pituitary tumour.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

