Essential Roles of PIEZO1 in Mammalian Cardiovascular System: From Development to Diseases
- PMID: 39272994
- PMCID: PMC11394449
- DOI: 10.3390/cells13171422
Essential Roles of PIEZO1 in Mammalian Cardiovascular System: From Development to Diseases
Abstract
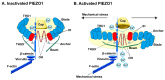
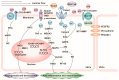
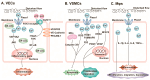
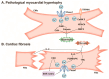
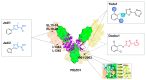
Mechanical force is the basis of cardiovascular development, homeostasis, and diseases. The perception and response of mechanical force by the cardiovascular system are crucial. However, the molecular mechanisms mediating mechanotransduction in the cardiovascular system are not yet understood. PIEZO1, a novel transmembrane mechanosensitive cation channel known for its regulation of touch sensation, has been found to be widely expressed in the mammalian cardiovascular system. In this review, we elucidate the role and mechanism of PIEZO1 as a mechanical sensor in cardiovascular development, homeostasis, and disease processes, including embryo survival, angiogenesis, cardiac development repair, vascular inflammation, lymphangiogenesis, blood pressure regulation, cardiac hypertrophy, cardiac fibrosis, ventricular remodeling, and heart failure. We further summarize chemical molecules targeting PIEZO1 for potential translational applications. Finally, we address the controversies surrounding emergent concepts and challenges in future applications.
Keywords: PIEZO1; cardiovascular system; chemical therapies; development; diseases; homeostasis; mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

