Understanding Macrophage Interaction with Antimony-Doped Tin Oxide Plasmonic Nanoparticles
- PMID: 39273038
- PMCID: PMC11394000
- DOI: 10.3390/cells13171468
Understanding Macrophage Interaction with Antimony-Doped Tin Oxide Plasmonic Nanoparticles
Abstract
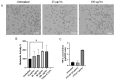
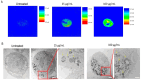
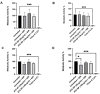
Antimony-doped tin oxide nanoparticles (ATO NPs) have emerged as a promising tool in biomedical applications, namely robust photothermal effects upon near-infrared (NIR) light exposure, enabling controlled thermal dynamics to induce spatial cell death. This study investigated the interplay between ATO NPs and macrophages, understanding cellular uptake and cytokine release. ATO NPs demonstrated biocompatibility with no impact on macrophage viability and cytokine secretion. These findings highlight the potential of ATO NPs for inducing targeted cell death in cancer treatments, leveraging their feasibility, unique NIR properties, and safe interactions with immune cells. ATO NPs offer a transformative platform with significant potential for future biomedical applications by combining photothermal capabilities and biocompatibility.
Keywords: antimony-doped tin oxide nanoparticles; cellular uptake; macrophages; near-infrared radiation; photothermal effects.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

