Class Effect Unveiled: PPARγ Agonists and MEK Inhibitors in Cancer Cell Differentiation
- PMID: 39273076
- PMCID: PMC11394433
- DOI: 10.3390/cells13171506
Class Effect Unveiled: PPARγ Agonists and MEK Inhibitors in Cancer Cell Differentiation
Abstract
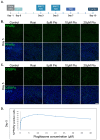
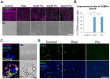
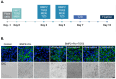
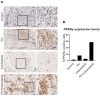
Epithelial-to-mesenchymal transition (EMT) plays a major role in breast cancer progression and the development of drug resistance. We have previously demonstrated a trans-differentiation therapeutic approach targeting invasive dedifferentiated cancer cells. Using a combination of PPARγ agonists and MEK inhibitors, we forced the differentiation of disseminating breast cancer cells into post-mitotic adipocytes. Utilizing murine breast cancer cells, we demonstrated a broad class effect of PPARγ agonists and MEK inhibitors in inducing cancer cell trans-differentiation into adipocytes. Both Rosiglitazone and Pioglitazone effectively induced adipogenesis in cancer cells, marked by PPARγ and C/EBPα upregulation, cytoskeleton rearrangement, and lipid droplet accumulation. All tested MEK inhibitors promoted adipogenesis in the presence of TGFβ, with Cobimetinib showing the most prominent effects. A metastasis ex vivo culture from a patient diagnosed with triple-negative breast cancer demonstrated a synergistic upregulation of PPARγ with the combination of Pioglitazone and Cobimetinib. Our results highlight the potential for new therapeutic strategies targeting cancer cell plasticity and the dedifferentiation phenotype in aggressive breast cancer subtypes. Combining differentiation treatments with standard therapeutic approaches may offer a strategy to overcome drug resistance.
Keywords: EMT; MEK inhibitor; PPARγ agonist; adipogenesis; breast cancer; differentiation therapy; thiazolidinediones.
Conflict of interest statement
Authors Vered Bar, Sara Aharon, Anna Kosenko and Adi Zundelevich were employed by the company Curesponse Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Curesponse Ltd. had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

