TNFα-Induced Inflammation Model-Evaluation of Concentration and Passage-Dependent Effects on Bovine Chondrocytes
- PMID: 39273085
- PMCID: PMC11395278
- DOI: 10.3390/ijms25179136
TNFα-Induced Inflammation Model-Evaluation of Concentration and Passage-Dependent Effects on Bovine Chondrocytes
Abstract
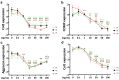
Inflammation models are widely used in the in vitro investigation of new therapeutic approaches for osteoarthritis. TNFα (tumor necrosis factor alpha) plays an important role in the inflammatory process. Current inflammation models lack uniformity and make comparisons difficult. Therefore, this study aimed to systematically investigate whether the effects of TNFα are concentration-dependent and whether chondrocyte expansion has an effect on the inflammatory model. Bovine chondrocytes were enzymatically isolated, expanded to passages 1-3, and transferred into a 3D pellet culture. Chondrocyte pellets were stimulated with recombinant bovine TNFα at different concentrations for 48 h to induce inflammation. Gene expression of anabolic (collagen 2, aggrecan, cartilage oligomeric protein (COMP)), catabolic (matrix metalloproteinases (MMP3, MMP13)), dedifferentiation (collagen 1) markers, inflammation markers (interleukin-6 (IL-6), nuclear factor kappa B (NFkB), cyclooxygenase-2 (COX), prostaglandin-E-synthase-2 (PTGES2)), and the apoptosis marker caspase 3 was determined. At the protein level, concentrations of IL-6, nitric oxide (NO), and sulfated glycosaminoglycans (GAG) were evaluated. Statistical analysis was performed using the independent t-test, and significance was defined as p < 0.05. In general, TNFα caused a decrease in anabolic markers and an increase in the expression of catabolic and inflammatory markers. There was a concentration-dependent threshold of 10 ng/mL to induce significant inflammatory effects. Most of the markers analyzed showed TNFα concentration-dependent effects (COMP, PRG4, AGN, Col1, MMP3, and NFkB). There was a statistical influence of selected gene expression markers from different passages on the TNFα chondrocyte inflammation model, including Col2, MMP13, IL-6, NFkB, COX2, and PTGES2. Considering the expression of collagen 2 and MMP3, passage 3 chondrocytes showed a higher sensitivity to TNFα stimulation compared to passages 1 and 2. On the other hand, MMP13, IL-6, NFkB, and caspase 3 gene expression were lower in P3 chondrocytes compared to the other passages. On the protein level, inflammatory effects showed a similar pattern, with cytokine effects starting at 10 ng/mL and differences between the passages. TNFα had a detrimental effect on cartilage, with a clear threshold observed at 10 ng/mL. Although TNFα effects showed concentration-dependent patterns, this was not consistent for all markers. The selected passage showed a clear influence, especially on inflammation markers. Further experiments were warranted to explore the effects of TNFα concentration and passage in long-term stimulation.
Keywords: TNFα; bovine; cartilage; chondrocyte; cytokine; inflammation model.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Jin Z., Wang D., Zhang H., Liang J., Feng X., Zhao J., Sun L. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: Results from the global burden of disease study 2017. Ann. Rheum. Dis. 2020;79:1014–1022. doi: 10.1136/annrheumdis-2020-217050. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

