BMP4-Induced Suppression of Breast Cancer Metastasis Is Associated with Inhibition of Cholesterol Biosynthesis
- PMID: 39273106
- PMCID: PMC11395556
- DOI: 10.3390/ijms25179160
BMP4-Induced Suppression of Breast Cancer Metastasis Is Associated with Inhibition of Cholesterol Biosynthesis
Abstract
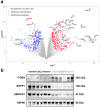
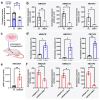
We reported previously that in preclinical models, BMP4 is a potent inhibitor of breast cancer metastasis and that high BMP4 protein levels predict favourable patient outcomes. Here, we analysed a breast cancer xenograft with or without enforced expression of BMP4 to gain insight into the mechanisms by which BMP4 suppresses metastasis. Transcriptomic analysis of cancer cells recovered from primary tumours and phosphoproteomic analyses of cancer cells exposed to recombinant BMP4 revealed that BMP4 inhibits cholesterol biosynthesis, with many genes in this biosynthetic pathway being downregulated by BMP4. The treatment of mice bearing low-BMP4 xenografts with a cholesterol-lowering statin partially mimicked the anti-metastatic activity of BMP4. Analysis of a cohort of primary breast cancers revealed a reduced relapse rate for patients on statin therapy if their tumours exhibited low BMP4 levels. These findings indicate that BMP4 may represent a predictive biomarker for the benefit of additional statin therapy in breast cancer patients.
Keywords: BMP4; breast cancer; cholesterol biosynthesis; metastasis; statins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Khan S.A., Zhao F., Goldstein L.J., Cella D., Basik M., Golshan M., Julian T.B., Pockaj B.A., Lee C.A., Razaq W., et al. Early Local Therapy for the Primary Site in De Novo Stage IV Breast Cancer: Results of a Randomized Clinical Trial (EA2108) J. Clin. Oncol. 2022;40:978–987. doi: 10.1200/JCO.21.02006. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

