The Contribution of Mast Cells to the Regulation of Elastic Fiber Tensometry in the Skin Dermis of Children with Marfan Syndrome
- PMID: 39273142
- PMCID: PMC11394836
- DOI: 10.3390/ijms25179191
The Contribution of Mast Cells to the Regulation of Elastic Fiber Tensometry in the Skin Dermis of Children with Marfan Syndrome
Abstract
Marfan syndrome (MFS) is a hereditary condition accompanied by disorders in the structural and regulatory properties of connective tissue, including elastic fibers, due to a mutation in the gene encodes for fibrillin-1 protein (FBN1 gene) and the synthesis of abnormal fibrillin-1 glycoprotein. Despite the high potential of mast cells (MCs) to remodel the extracellular matrix (ECM), their pathogenetic significance in MFS has not been considered yet. The group of patients with Marfan syndrome included two mothers and five children (three girls aged 4, 11, and 11 and two boys aged 12 and 13). Normal skin was examined in two children aged 11 and 12. Histochemical, monoplex, and multiplex immunohistochemical techniques; combined protocols of simultaneous histochemical and immunohistochemical staining (the results of staining were assessed using light, epifluorescence, and confocal microscopy); and bioinformatics algorithms for the quantitative analysis of detected targets were used to evaluate mast cells and their relationship with other cells from extracellular structures in the skin dermis. Analysis of the skin MC population in children with Marfan syndrome revealed a considerably increased number of intra-organic populations with the preservation of the specific Tryptase+Chymase+CPA3+ protease profile typical of the skin. The features of the MC histotopography phenotype in MFS consisted of closer colocalization with elastic fibers, smooth muscle cells, and fibroblasts. MCs formed many intradermal clusters that synchronized the activity of cell functions in the stromal landscape of the tissue microenvironment with the help of spatial architectonics, including the formation of cell chains and the creation of fibrous niches. In MCs, the expression of specific proteases, TGF-β, and heparin increased, with targeted secretion of biologically active substances relative to the dermal elastic fibers, which had specific structural features in MFS, including abnormal variability in thickness along their entire length, alternating thickened and thinned areas, and uneven surface topography. This paper discusses the potential role of MCs in strain analysis (tensometry) of the tissue microenvironment in MFS. Thus, the quantitative and qualitative rearrangements of the skin MC population in MFS are aimed at altering the stromal landscape of the connective tissue. The results obtained should be taken into account when managing clinical signs of MFS manifested in other pathogenetically critical structures of internal organs, including the aorta, tendons, cartilage, and parenchymal organs.
Keywords: Marfan syndrome; carboxypeptidase A3; chymase; elastic fibers; heparin; mast cells; skin; tensometry extracellular matrix remodeling; tryptase.
Conflict of interest statement
The authors declare no conflicts of interest, and they confirm that this paper has not been and will not be published in whole or in part in any other journal and is not under consideration for publication elsewhere. This manuscript was approved by all authors.
Figures
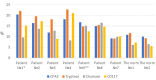
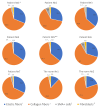
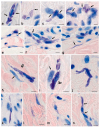

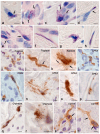



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

