Lipid Peroxidation Regulators GPX4 and FSP1 as Prognostic Markers and Therapeutic Targets in Advanced Gastric Cancer
- PMID: 39273151
- PMCID: PMC11395505
- DOI: 10.3390/ijms25179203
Lipid Peroxidation Regulators GPX4 and FSP1 as Prognostic Markers and Therapeutic Targets in Advanced Gastric Cancer
Abstract
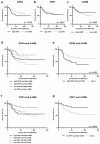
Gastric cancer is one of the most common cancers worldwide, and new therapeutic strategies are urgently needed. Ferroptosis is an intracellular iron-dependent cell death induced by the accumulation of lipid peroxidation, a mechanism different from conventional apoptosis and necrosis. Therefore, induction of ferroptosis is expected to be a new therapeutic strategy. Glutathione peroxidase 4 (GPX4) and ferroptosis suppressor protein 1 (FSP1) have been identified as the major inhibitors of ferroptosis. Herein, we performed immunohistochemistry for GPX4, FSP1, and 4-HNE using tissues from patients with gastric cancer and investigated the relationship between these factors and prognosis. Patients with high GPX4 expression or high GPX4 expression and low 4-HNE accumulation tended to have a poor prognosis (p = 0.036, 0.023), whereas those with low FSP1 expression and high 4-HNE accumulation had a good prognosis (p = 0.033). The synergistic induction of cell death by inhibiting GPX4 and FSP1 in vitro was also observed, indicating that the cell death was non-apoptotic. Our results indicate that the expression and accumulation of lipid peroxidation-related factors play an important role in the clinicopathological significance of gastric cancer and that novel therapeutic strategies targeting GPX4 and FSP1 may be effective in treating patients with gastric cancer who have poor prognosis.
Keywords: chemotherapy; ferroptosis; gastric cancer; lipid peroxidation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

