Lectin-Based Fluorescent Comparison of Glycan Profile-FDA Validation to Expedite Approval of Biosimilars
- PMID: 39273189
- PMCID: PMC11395676
- DOI: 10.3390/ijms25179240
Lectin-Based Fluorescent Comparison of Glycan Profile-FDA Validation to Expedite Approval of Biosimilars
Abstract
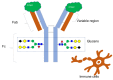
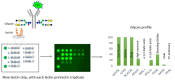
Glycan profile comparisons are one of the most tedious analytical exercises for establishing compliance with recombinant therapeutic protein batches. Based on its intensive research, the FDA has confirmed that lectin array binding with fluorescent monitoring is the fastest and most reliable method for profile comparisons. Using a database of over 150 biological products expressed in nine diverse mammalian cell systems, the FDA immobilized 74 lectins to study their binding using fluorescently labeled glycoproteins. The FDA identified nine distinct lectins from a custom-designed lectin microarray: rPhoSL, rOTH3, RCA120, rMan2, MAL_I, rPSL1a, PHAE, rMOA, and PHALs, which detect core fucose, terminal GlcNAc, terminal β-galactose, high mannose, α-2,3-linked sialic acids, α-2,6-linked sialic acids, bisecting GlcNAc, terminal α-galactose, and triantennary structures, respectively. This method can be used for screening and routine testing and to monitor batch-to-batch variability of therapeutic proteins, including establishing analytical similarity as a crucial part of biosimilar development.
Keywords: FDA; antibodies; biosimilars; fluorescent monitoring; lectins; microarray.
Conflict of interest statement
The authors are developers of biosimilar products.
Figures
References
-
- FDA The Purple Book. [(accessed on 2 July 2024)]; Available online: https://purplebooksearch.fda.gov/2024.
-
- Goding J.W., editor. Monoclonal Antibodies: Principles and Practice. 3rd ed. Elsevier; Amsterdam, The Netherlands: 1996.
-
- National Cancer Institute (U.S.) Antigen. [(accessed on 2 July 2024)]; Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/antige....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

