Staphylococcus aureus Stress Response to Bicarbonate Depletion
- PMID: 39273203
- PMCID: PMC11394868
- DOI: 10.3390/ijms25179251
Staphylococcus aureus Stress Response to Bicarbonate Depletion
Abstract
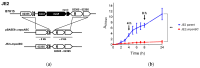
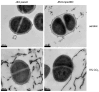
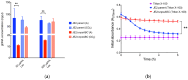
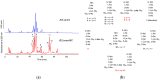
Bicarbonate and CO2 are essential substrates for carboxylation reactions in bacterial central metabolism. In Staphylococcus aureus, the bicarbonate transporter, MpsABC (membrane potential-generating system) is the only carbon concentrating system. An mpsABC deletion mutant can hardly grow in ambient air. In this study, we investigated the changes that occur in S. aureus when it suffers from CO2/bicarbonate deficiency. Electron microscopy revealed that ΔmpsABC has a twofold thicker cell wall thickness compared to the parent strain. The mutant was also substantially inert to cell lysis induced by lysostaphin and the non-ionic surfactant Triton X-100. Mass spectrometry analysis of muropeptides revealed the incorporation of alanine into the pentaglycine interpeptide bridge, which explains the mutant's lysostaphin resistance. Flow cytometry analysis of wall teichoic acid (WTA) glycosylation patterns revealed a significantly lower α-glycosylated and higher ß-glycosylated WTA, explaining the mutant's increased resistance towards Triton X-100. Comparative transcriptome analysis showed altered gene expression profiles. Autolysin-encoding genes such as sceD, a lytic transglycosylase encoding gene, were upregulated, like in vancomycin-intermediate S. aureus mutants (VISA). Genes related to cell wall-anchored proteins, secreted proteins, transporters, and toxins were downregulated. Overall, we demonstrate that bicarbonate deficiency is a stress response that causes changes in cell wall composition and global gene expression resulting in increased resilience to cell wall lytic enzymes and detergents.
Keywords: MpsABC; Staphylococcus aureus; bicarbonate transporter; cell wall; peptidoglycan; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

