Endothelial Cell-Derived Soluble CD200 Determines the Ability of Immune Cells to Cross the Blood-Brain Barrier
- PMID: 39273210
- PMCID: PMC11395061
- DOI: 10.3390/ijms25179262
Endothelial Cell-Derived Soluble CD200 Determines the Ability of Immune Cells to Cross the Blood-Brain Barrier
Abstract
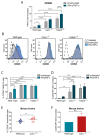
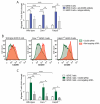
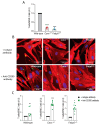
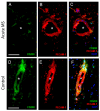
The infiltration of immune cells into the central nervous system mediates the development of autoimmune neuroinflammatory diseases. We previously showed that the loss of either Fabp5 or calnexin causes resistance to the induction of experimental autoimmune encephalomyelitis (EAE) in mice, an animal model of multiple sclerosis (MS). Here we show that brain endothelial cells lacking either Fabp5 or calnexin have an increased abundance of cell surface CD200 and soluble CD200 (sCD200) as well as decreased T-cell adhesion. In a tissue culture model of the blood-brain barrier, antagonizing the interaction of CD200 and sCD200 with T-cell CD200 receptor (CD200R1) via anti-CD200 blocking antibodies or the RNAi-mediated inhibition of CD200 production by endothelial cells increased T-cell adhesion and transmigration across monolayers of endothelial cells. Our findings demonstrate that sCD200 produced by brain endothelial cells regulates immune cell trafficking through the blood-brain barrier and is primarily responsible for preventing activated T-cells from entering the brain.
Keywords: CD200; CD200R1; brain endothelial cells; calnexin; fatty acid binding protein 5; neurodegenerative diseases; sCD200.
Conflict of interest statement
Author Paul Eggleton was employed by the company Revolo Biotherapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Kolliker-Frers R., Udovin L., Otero-Losada M., Kobiec T., Herrera M.I., Palacios J., Razzitte G., Capani F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediators Inflamm. 2021;2021:9999146. doi: 10.1155/2021/9999146. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

