Role of Nitric Oxide Synthases in Respiratory Health and Disease: Insights from Triple Nitric Oxide Synthases Knockout Mice
- PMID: 39273265
- PMCID: PMC11395504
- DOI: 10.3390/ijms25179317
Role of Nitric Oxide Synthases in Respiratory Health and Disease: Insights from Triple Nitric Oxide Synthases Knockout Mice
Abstract
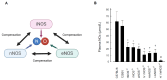
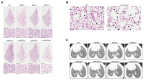
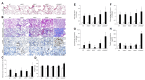
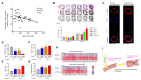
The system of nitric oxide synthases (NOSs) is comprised of three isoforms: nNOS, iNOS, and eNOS. The roles of NOSs in respiratory diseases in vivo have been studied by using inhibitors of NOSs and NOS-knockout mice. Their exact roles remain uncertain, however, because of the non-specificity of inhibitors of NOSs and compensatory up-regulation of other NOSs in NOS-KO mice. We addressed this point in our triple-n/i/eNOSs-KO mice. Triple-n/i/eNOSs-KO mice spontaneously developed pulmonary emphysema and displayed exacerbation of bleomycin-induced pulmonary fibrosis as compared with wild-type (WT) mice. Triple-n/i/eNOSs-KO mice exhibited worsening of hypoxic pulmonary hypertension (PH), which was reversed by treatment with sodium nitrate, and WT mice that underwent triple-n/i/eNOSs-KO bone marrow transplantation (BMT) also showed aggravation of hypoxic PH compared with those that underwent WT BMT. Conversely, ovalbumin-evoked asthma was milder in triple-n/i/eNOSs-KO than WT mice. These results suggest that the roles of NOSs are different in different pathologic states, even in the same respiratory diseases, indicating the diversity of the roles of NOSs. In this review, we describe these previous studies and discuss the roles of NOSs in respiratory health and disease. We also explain the current state of development of inorganic nitrate as a new drug for respiratory diseases.
Keywords: asthma; bone marrow; mice; nitrate; nitric oxide; nitric oxide synthases; pulmonary emphysema; pulmonary fibrosis; pulmonary hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

