Lipodystrophic Laminopathies: From Dunnigan Disease to Progeroid Syndromes
- PMID: 39273270
- PMCID: PMC11395136
- DOI: 10.3390/ijms25179324
Lipodystrophic Laminopathies: From Dunnigan Disease to Progeroid Syndromes
Abstract
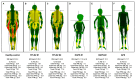
Lipodystrophic laminopathies are a group of ultra-rare disorders characterised by the presence of pathogenic variants in the same gene (LMNA) and other related genes, along with an impaired adipose tissue pattern and other features that are specific of each of these disorders. The most fascinating traits include their complex genotype-phenotype associations and clinical heterogeneity, ranging from Dunnigan disease, in which the most relevant feature is precisely adipose tissue dysfunction and lipodystrophy, to the other laminopathies affecting adipose tissue, which are also characterised by the presence of signs of premature ageing (Hutchinson Gilford-progeria syndrome, LMNA-atypical progeroid syndrome, mandibuloacral dysplasia types A and B, Nestor-Guillermo progeria syndrome, LMNA-associated cardiocutaneous progeria). This raises several questions when it comes to understanding how variants in the same gene can lead to similar adipose tissue disturbances and, at the same time, to such heterogeneous phenotypes and variable degrees of metabolic abnormalities. The present review aims to gather the molecular basis of adipose tissue impairment in lipodystrophic laminopathies, their main clinical aspects and recent therapeutic strategies. In addition, it also summarises the key aspects for their differential diagnosis.
Keywords: Dunnigan disease; FPLD; Hutchinson-Gilford progeria syndrome; Nestor-Guillermo progeria syndrome; adipose tissue; atypical progeroid syndrome; laminopathies; lipodystrophy; mandibuloacral dysplasia; progeria.
Conflict of interest statement
D.A.-V. has received fees from Amryt Pharmaceuticals and Regeneron Pharmaceuticals for scientific advice, travel, conference registration and research grants. The rest of the authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

