Deciphering DNA Methylation in Gestational Diabetes Mellitus: Epigenetic Regulation and Potential Clinical Applications
- PMID: 39273309
- PMCID: PMC11394902
- DOI: 10.3390/ijms25179361
Deciphering DNA Methylation in Gestational Diabetes Mellitus: Epigenetic Regulation and Potential Clinical Applications
Abstract
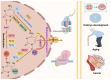
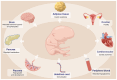
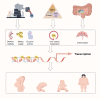
Gestational diabetes mellitus (GDM) represents a prevalent complication during pregnancy, exerting both short-term and long-term impacts on maternal and offspring health. This review offers a comprehensive outline of DNA methylation modifications observed in various maternal and offspring tissues affected by GDM, emphasizing the intricate interplay between DNA methylation dynamics, gene expression, and the pathogenesis of GDM. Furthermore, it explores the influence of environmental pollutants, maternal nutritional supplementation, and prenatal gut microbiota on GDM development through alterations in DNA methylation profiles. Additionally, this review summarizes recent advancements in DNA methylation-based diagnostics and predictive models in early GDM detection and risk assessment for subsequent type 2 diabetes. These insights contribute significantly to our understanding of the epigenetic mechanisms underlying GDM development, thereby enhancing maternal and fetal health outcomes and advocating further efforts in this field.
Keywords: DNA methylation; epigenetics; gestational diabetes mellitus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Fat mass and obesity-associated (FTO) gene epigenetic modifications in gestational diabetes: new insights and possible pathophysiological connections.Acta Diabetol. 2021 Aug;58(8):997-1007. doi: 10.1007/s00592-020-01668-5. Epub 2021 Mar 20. Acta Diabetol. 2021. PMID: 33743080 Free PMC article.
-
Longitudinal DNA methylation profiles in saliva of offspring from mothers with gestational diabetes: associations with early childhood growth patterns.Cardiovasc Diabetol. 2025 Jan 13;24(1):15. doi: 10.1186/s12933-024-02568-6. Cardiovasc Diabetol. 2025. PMID: 39806399 Free PMC article.
-
Epigenetic Changes in Gestational Diabetes Mellitus.Int J Mol Sci. 2021 Jul 17;22(14):7649. doi: 10.3390/ijms22147649. Int J Mol Sci. 2021. PMID: 34299269 Free PMC article. Review.
-
Epigenetics and gestational diabetes: a review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction.Diabetologia. 2019 Dec;62(12):2171-2178. doi: 10.1007/s00125-019-05011-8. Epub 2019 Oct 17. Diabetologia. 2019. PMID: 31624900 Free PMC article. Review.
-
The Impact of Gestational Diabetes on Kidney Development: is There an Epigenetic Link?Curr Diab Rep. 2024 Dec 18;25(1):13. doi: 10.1007/s11892-024-01569-9. Curr Diab Rep. 2024. PMID: 39690358 Free PMC article. Review.
Cited by
-
DNA methylation patterns and predictive models for metabolic disease risk in offspring of gestational diabetes mellitus.Diabetol Metab Syndr. 2025 May 2;17(1):147. doi: 10.1186/s13098-025-01707-7. Diabetol Metab Syndr. 2025. PMID: 40312441 Free PMC article.
References
-
- Diabetes Data Report 2000—2045. International Diabetes Federation; Amsterdam, The Netherlands: 2021.
-
- Shah N.S., Wang M.C., Freaney P.M., Perak A.M., Carnethon M.R., Kandula N.R., Gunderson E.P., Bullard K.M., Grobman W.A., O’Brien M.J., et al. Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US, 2011-2019. JAMA. 2021;326:660–669. doi: 10.1001/jama.2021.7217. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

