A Cautionary Tale of Hypertrophic Cardiomyopathy-From "Benign" Left Ventricular Hypertrophy to Stroke, Atrial Fibrillation, and Molecular Genetic Diagnostics: A Case Report and Review of Literature
- PMID: 39273332
- PMCID: PMC11395475
- DOI: 10.3390/ijms25179385
A Cautionary Tale of Hypertrophic Cardiomyopathy-From "Benign" Left Ventricular Hypertrophy to Stroke, Atrial Fibrillation, and Molecular Genetic Diagnostics: A Case Report and Review of Literature
Abstract
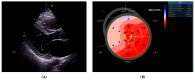
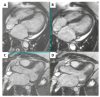
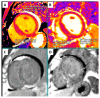
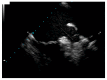
This case report concerns a 48-year-old man with a history of ischemic stroke at the age of 41 who reported cardiac hypertrophy, registered in his twenties when explained by increased physical activity. Family history was positive for a mother with permanent atrial fibrillation from her mid-thirties. At the age of 44, he had a first episode of persistent atrial fibrillation, accompanied by left atrial thrombosis while on a direct oral anticoagulant. He presented at our clinic at the age of 45 with another episode of persistent atrial fibrillation and decompensated heart failure. Echocardiography revealed a dilated left atrium, reduced left ventricular ejection fraction, and an asymmetric left ventricular hypertrophy. Cardiac magnetic resonance was positive for a cardiomyopathy with diffuse fibrosis, while slow-flow phenomenon was present on coronary angiography. Genetic testing by whole-exome sequencing revealed three variants in the patient, c.309C > A, p.His103Gln in the ACTC1 gene, c.116T > G, p.Leu39Ter in the PLN gene, and c.5827C > T, p.His1943Tyr in the SCN5A gene, the first two associated with hypertrophic cardiomyopathy and the latter possibly with familial atrial fibrillation. This case illustrates the need for advanced diagnostics in unexplained left ventricular hypertrophy, as hypertrophic cardiomyopathy is often overlooked, leading to potentially debilitating health consequences.
Keywords: ACTC1; PLN; SCN5A; atrial fibrillation; cardiac magnetic resonance; coronary slow-flow phenomenon; genetic testing; hypertrophic cardiomyopathy; left ventricular hypertrophy; whole-exome sequencing.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Maron B.J., Gardin J.M., Flack J.M., Gidding S.S., Kurosaki T.T., Bild D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. doi: 10.1161/01.CIR.92.4.785. - DOI - PubMed
-
- Gerull B., Klaassen S., Brodehl A. The Genetic Landscape of Cardiomyopathies. In: Erdmann J., Moretti A., editors. Genetic Causes of Cardiac Disease. Cardiac and Vascular Biology. Volume 7. Springer; Cham, Switzerland: 2019. - DOI
-
- Ingles J., Burns C., Bagnall R.D., Lam L., Yeates L., Sarina T., Puranik R., Briffa T., Atherton J.J., Driscoll T., et al. Nonfamilial Hypertrophic Cardiomyopathy: Prevalence, Natural History, and Clinical Implications. Circ. Cardiovasc. Genet. 2017;10:e001620. doi: 10.1161/CIRCGENETICS.116.001620. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

