Analytical Investigation of the Profile of Human Chorionic Gonadotropin in Highly Purified Human Menopausal Gonadotrophin Preparations
- PMID: 39273352
- PMCID: PMC11395176
- DOI: 10.3390/ijms25179405
Analytical Investigation of the Profile of Human Chorionic Gonadotropin in Highly Purified Human Menopausal Gonadotrophin Preparations
Abstract
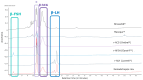
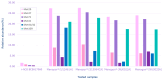
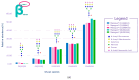
Highly purified human menopausal gonadotropin (HP-hMG [Menopur®, Ferring Pharmaceuticals, Saint-Prex, Switzerland]) contains a 1:1 ratio of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This analysis aimed to assess gonadotropin (FSH, LH and hCG) abundance in HP-hMG and clarify the source of hCG by assessing the presence of sulfated glycans, which are diagnostic for pituitary hCG forms due to their distinct glycosylation patterns. Additionally, the purity of each sample, their specific components, and their oxidation levels were assessed. HP-hMG samples (three of Menopur® and two of Menogon® Ferring Pharmaceuticals, Saint-Prex, Switzerland) were included in the current analyses. Brevactid® (urinary hCG; Ferring Pharmaceuticals, Saint-Prex, Switzerland) and Ovidrel® (recombinant hCG; Merck KGaA, Darmstadt, Germany) were used as control samples. Glycopeptide mapping and analysis of impurities were carried out by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Oxidation was assessed through reducing peptide mapping using LC-MS/MS. The FSH and LH in the HP-hMG samples showed sulfated glycans, while no signals of sulfated glycopeptides were detected on any site of the beta subunit of hCG. HP-hMG test samples presented the same hCG glycan distribution as the control sample (placental hCG, Brevactid®) extracted from the urine of pregnant women, suggesting a non-pituitary source of hCG. Protein impurities were estimated to constitute approximately 20-30% of the entire HP-hMG protein content in the test samples. More than 200 non-gonadotropin proteins were identified in the HP-hMG test samples, of which several were involved in embryonic development or pregnancy. The alpha subunit of the tested samples was strongly oxidized, with a relative abundance of 20% of the total gonadotropin content. Without taking into account all the protein impurities, the beta subunit of LH was detected only in traces (0.9-1.2%) in all tested HP-HMG samples, confirming the data obtained by intact molecule analysis, while high levels of beta hCG (18-47%) were observed. Advanced molecular analysis of HP-hMG indicates a primarily placental origin of hCG, as evidenced by the absence of hCG sulfated glycans and the predominance of placental non-sulfated hCG in LH activity. The analysis revealed 20-30% of protein impurities and a significant presence of oxidized forms in the HP-hMG samples. These findings are critical for understanding the quality, safety, and clinical profile of HP-hMG.
Keywords: glycosylation profiling; highly purified human menopausal gonadotropin; luteinizing hormone; placental human chorionic gonadotropin.
Conflict of interest statement
Angela Capolupo, Monica Lispi, Sofia Petrocchi, Maura Melchiorre, Patrizia Simone, and Gabriella Angiuoni are employed by Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA. Angelo Palmese was employed by Merck KGaA at the time of the study. Thomas D’Hooghe and Susana Montenegro are employed by Merck KGaA, Darmstadt, Germany. Sesh Sunkara reports reception of payment or honoraria for lectures from Merck, Ferring, and MSD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures





References
-
- Giudice E., Crisci C., Altarocca V., O’Brien M. Characterisation of a partially purified human menopausal gonadotropin preparation. J. Clin. Res. 2001;4:27–33.
-
- Bassett R., Ceccarelli D., Crisci C., di Tria A.M., Mancinelli M., Martelli F., Mendola D., Pezzotti A. Comparative analytical characterization of two commercial human follicle-stimulating hormones extracted from human urine. Curr. Med. Res. Opin. 2005;21:899–905. doi: 10.1185/030079905X48410. - DOI - PubMed
-
- Menopur 75 IU UK SmPC (November 2019) [(accessed on 25 June 2024)]. Available online: https://www.medicines.org.uk/emc/product/1294/smpc#gref.
-
- Patent WO 00/63248. 2000. [(accessed on 25 June 2024)]. Available online: https://patents.google.com/patent/WO2005063811A1/en.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

