Expression of MicroRNAs in Adults with Celiac Disease: A Narrative Review
- PMID: 39273359
- PMCID: PMC11395070
- DOI: 10.3390/ijms25179412
Expression of MicroRNAs in Adults with Celiac Disease: A Narrative Review
Abstract
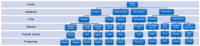
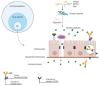
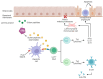
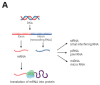
Celiac disease (CD) is an immune-mediated enteropathy triggered by the ingestion of proline- and glutamine-rich proteins, widely termed "gluten", in genetically susceptible individuals. CD induces an altered immune response that leads to chronic inflammation and duodenal mucosal damage. Currently, there are no specific tests for the accurate diagnosis of CD, and no drugs are available to treat this condition. The only available treatment strategy is lifelong adherence to a gluten-free diet. However, some studies have investigated the involvement of microRNAs (miRNAs) in CD pathogenesis. miRNAs are small noncoding ribonucleic acid molecules that regulate gene expression. Despite the growing number of studies on the role of miRNAs in autoimmune disorders, data on miRNAs and CD are scarce. Therefore, this study aimed to perform a literature review to summarize CD, miRNAs, and the potential interactions between miRNAs and CD in adults. This review shows that miRNA expression can suppress or stimulate pathways related to CD pathogenesis by regulating cell proliferation and differentiation, regulatory T-cell development, innate immune response, activation of the inflammatory cascade, focal adhesion, T-cell commitment, tissue transglutaminase synthesis, and cell cycle. Thus, identifying miRNAs and their related effects on CD could open new possibilities for diagnosis, prognosis, and follow-up of biomarkers.
Keywords: biomarkers; celiac disease; gluten-free diet; immune response; microRNAs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Paolini A., Sarshar M., Felli C., Bruno S.P., Rostami-Nejad M., Ferretti F., Masotti A., Baldassarre A. Biomarkers to monitor adherence to gluten-free diet by celiac disease patients: Gluten immunogenic peptides and urinary miRNAs. Foods. 2022;11:1380. doi: 10.3390/foods11101380. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

