Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications
- PMID: 39273362
- PMCID: PMC11395326
- DOI: 10.3390/ijms25179416
Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications
Abstract
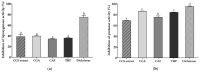
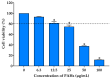
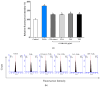
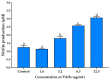
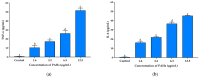
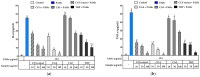
Airborne particulate matter (PM) contains polycyclic aromatic hydrocarbons (PAHs) as primary toxic components, causing oxidative damage and being associated with various inflammatory skin pathologies such as premature aging, atopic dermatitis, and psoriasis. Coffee cherry pulp (CCS) extract, rich in chlorogenic acid, caffeine, and theophylline, has demonstrated strong antioxidant properties. However, its specific anti-inflammatory effects and ability to protect macrophages against PAH-induced inflammation remain unexplored. Thus, this study aimed to evaluate the anti-inflammatory properties of CCS extract on RAW 264.7 macrophage cells exposed to atmospheric PAHs, compared to chlorogenic acid (CGA), caffeine (CAF), and theophylline (THP) standards. The CCS extract was assessed for its impact on the production of nitric oxide (NO) and expression of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Results showed that CCS extract exhibited significant antioxidant activities and effectively inhibited protease and lipoxygenase (LOX) activities. The PAH induced the increase in intracellular reactive oxygen species, NO, TNF-α, IL-6, iNOS, and COX-2, which were markedly suppressed by CCS extract in a dose-dependent manner, comparable to the effects of chlorogenic acid, caffeine, and theophylline. In conclusion, CCS extract inhibits PAH-induced inflammation by reducing pro-inflammatory cytokines and reactive oxygen species (ROS) production in RAW 264.7 cells. This effect is likely due to the synergistic effects of its bioactive compounds. Chlorogenic acid showed strong antioxidant and anti-inflammatory activities, while caffeine and theophylline enhanced anti-inflammatory activity. CCS extract did not irritate the hen's egg chorioallantoic membrane. Therefore, CCS extract shows its potential as a promising cosmeceutical ingredient for safely alleviating inflammatory skin diseases caused by air pollution.
Keywords: anti-inflammation; caffeine; chlorogenic acid; coffee cherry pulp; cyclooxygenase-2; inducible nitric oxide synthase; interleukin-6; pollution; polycyclic aromatic hydrocarbons; theophylline; tumor necrosis factor-alpha.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts.Foods. 2023 Nov 28;12(23):4292. doi: 10.3390/foods12234292. Foods. 2023. PMID: 38231740 Free PMC article.
-
Red Bean Extract Inhibits Lipopolysaccharide-Induced Inflammation and H₂O₂-Induced Oxidative Stress in RAW 264.7 Macrophages.J Med Food. 2015 Jul;18(7):724-30. doi: 10.1089/jmf.2014.3353. Epub 2015 Apr 14. J Med Food. 2015. PMID: 25871313
-
Anti-inflammatory and antioxidant effects of MOK, a polyherbal extract, on lipopolysaccharide‑stimulated RAW 264.7 macrophages.Int J Mol Med. 2019 Jan;43(1):26-36. doi: 10.3892/ijmm.2018.3937. Epub 2018 Oct 16. Int J Mol Med. 2019. PMID: 30365058 Free PMC article.
-
Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds.Phytomedicine. 2014 Jan 15;21(2):164-71. doi: 10.1016/j.phymed.2013.08.008. Epub 2013 Sep 14. Phytomedicine. 2014. PMID: 24041614 Review.
-
Evidence that the aryl hydrocarbon receptor orchestrates oxinflammatory responses and contributes to airborne particulate matter-induced skin aging.Free Radic Biol Med. 2025 Jun;233:264-278. doi: 10.1016/j.freeradbiomed.2025.03.040. Epub 2025 Mar 27. Free Radic Biol Med. 2025. PMID: 40157462 Review.
Cited by
-
Protective Effects of Xanthorrhizol-Rich Extracts Against PM-Induced Skin Damage in Human Keratinocytes and 3D-Reconstructed Skin Models.Pharmaceuticals (Basel). 2025 May 28;18(6):808. doi: 10.3390/ph18060808. Pharmaceuticals (Basel). 2025. PMID: 40573205 Free PMC article.
-
Development and Evaluation of Anti-Pollution Film-Forming Facial Spray Containing Coffee Cherry Pulp Extract.Pharmaceutics. 2025 Mar 12;17(3):360. doi: 10.3390/pharmaceutics17030360. Pharmaceutics. 2025. PMID: 40143024 Free PMC article.
References
-
- Seok J.K., Lee J.W., Kim Y.M., Boo Y.C. Punicalagin and (-)-epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin. Pharmacol. Physiol. 2018;31:134–143. doi: 10.1159/000487400. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

