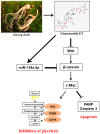
Anti-Warburg Mechanism of Ginsenoside F2 in Human Cervical Cancer Cells via Activation of miR193a-5p and Inhibition of β-Catenin/c-Myc/Hexokinase 2 Signaling Axis
- PMID: 39273365
- PMCID: PMC11394963
- DOI: 10.3390/ijms25179418
Anti-Warburg Mechanism of Ginsenoside F2 in Human Cervical Cancer Cells via Activation of miR193a-5p and Inhibition of β-Catenin/c-Myc/Hexokinase 2 Signaling Axis
Abstract
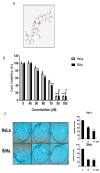
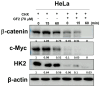
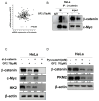
Though Ginsenoside F2 (GF2), a protopanaxadiol saponin from Panax ginseng, is known to have an anticancer effect, its underlying mechanism still remains unclear. In our model, the anti-glycolytic mechanism of GF2 was investigated in human cervical cancer cells in association with miR193a-5p and the β-catenin/c-Myc/Hexokinase 2 (HK2) signaling axis. Here, GF2 exerted significant cytotoxicity and antiproliferation activity, increased sub-G1, and attenuated the expression of pro-Poly (ADPribose) polymerase (pro-PARP) and pro-cysteine aspartyl-specific protease (procaspase3) in HeLa and SiHa cells. Consistently, GF2 attenuated the expression of Wnt, β-catenin, and c-Myc and their downstream target genes such as HK2, pyruvate kinase isozymes M2 (PKM2), and lactate dehydrogenase A (LDHA), along with a decreased production of glucose and lactate in HeLa and SiHa cells. Moreover, GF2 suppressed β-catenin and c-Myc stability in the presence and absence of cycloheximide in HeLa cells, respectively. Additionally, the depletion of β-catenin reduced the expression of c-Myc and HK2 in HeLa cells, while pyruvate treatment reversed the ability of GF2 to inhibit β-catenin, c-Myc, and PKM2 in GF2-treated HeLa cells. Notably, GF2 upregulated the expression of microRNA139a-5p (miR139a-5p) in HeLa cells. Consistently, the miR139a-5p mimic enhanced the suppression of β-catenin, c-Myc, and HK2, while the miR193a-5p inhibitor reversed the ability of GF2 to attenuate the expression of β-catenin, c-Myc, and HK2 in HeLa cells. Overall, these findings suggest that GF2 induces apoptosis via the activation of miR193a-5p and the inhibition of β-catenin/c-Myc/HK signaling in cervical cancer cells.
Keywords: apoptosis; cervical cancer; ginsenoside F2; glycolysis; miR193a-5p.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

