Advancing 3Rs: The Mouse Estrus Detector (MED) as a Low-Stress, Painless, and Efficient Tool for Estrus Determination in Mice
- PMID: 39273375
- PMCID: PMC11395264
- DOI: 10.3390/ijms25179429
Advancing 3Rs: The Mouse Estrus Detector (MED) as a Low-Stress, Painless, and Efficient Tool for Estrus Determination in Mice
Abstract
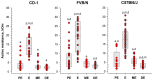
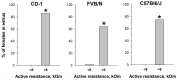
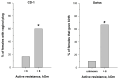
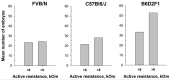
Determining the estrous cycle stages in mice is essential for optimizing breeding strategies, synchronizing experimental timelines, and facilitating studies in behavior, drug testing, and genetics. It is critical for reducing the production of genetically unmodified offspring in the generation and investigation of genetically modified animal models. An accurate detection of the estrus cycle is particularly relevant in the context of the 3Rs-Replacement, Reduction, and Refinement. The estrous cycle, encompassing the reproductive phases of mice, is key to refining experimental designs and addressing ethical issues related to the use of animals in research. This study presents results from two independent laboratories on the efficacy of the Mouse Estrus Detector (MED) from ELMI Ltd. (Latvia) for the accurate determination of the estrus phase. The female mice of five strains/stocks (CD1, FVB/N, C57Bl6/J, B6D2F1, and Swiss) were used. The results showed that the MEDProTM is a low-traumatic, simple, rapid, and painless method of estrus detection that supports the principles of the 3Rs. The use of the MEDProTM for estrus detection in mice caused minimal stress, enhanced mating efficiency, facilitated an increase in the number of embryos for in vitro fertilization, and allowed the production of the desired number of foster animals.
Keywords: 3Rs; female mice estrous cycle; mouse estrus detector; vaginal wall active resistance.
Conflict of interest statement
Dr. Dmitriy D. Merkulovs is co-inventor of patent LV15278B. The other co-authors have no conflict of interest to declare. ELMI Ltd. (Latvia) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources

