Non-Muscle Myosin II A: Friend or Foe in Cancer?
- PMID: 39273383
- PMCID: PMC11395477
- DOI: 10.3390/ijms25179435
Non-Muscle Myosin II A: Friend or Foe in Cancer?
Abstract
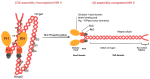
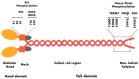
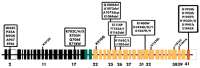
Non-muscle myosin IIA (NM IIA) is a motor protein that belongs to the myosin II family. The myosin heavy chain 9 (MYH9) gene encodes the heavy chain of NM IIA. NM IIA is a hexamer and contains three pairs of peptides, which include the dimer of heavy chains, essential light chains, and regulatory light chains. NM IIA is a part of the actomyosin complex that generates mechanical force and tension to carry out essential cellular functions, including adhesion, cytokinesis, migration, and the maintenance of cell shape and polarity. These functions are regulated via light and heavy chain phosphorylation at different amino acid residues. Apart from physiological functions, NM IIA is also linked to the development of cancer and genetic and neurological disorders. MYH9 gene mutations result in the development of several autosomal dominant disorders, such as May-Hegglin anomaly (MHA) and Epstein syndrome (EPS). Multiple studies have reported NM IIA as a tumor suppressor in melanoma and head and neck squamous cell carcinoma; however, studies also indicate that NM IIA is a critical player in promoting tumorigenesis, chemoradiotherapy resistance, and stemness. The ROCK-NM IIA pathway regulates cellular movement and shape via the control of cytoskeletal dynamics. In addition, the ROCK-NM IIA pathway is dysregulated in various solid tumors and leukemia. Currently, there are very few compounds targeting NM IIA, and most of these compounds are still being studied in preclinical models. This review provides comprehensive evidence highlighting the dual role of NM IIA in multiple cancer types and summarizes the signaling networks involved in tumorigenesis. Furthermore, we also discuss the role of NM IIA as a potential therapeutic target with a focus on the ROCK-NM IIA pathway.
Keywords: MYH9; motor protein; myosin IIA; non-muscle myosin IIA; tumorigenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

