Enhancing Therapeutic Efficacy of FLT3 Inhibitors with Combination Therapy for Treatment of Acute Myeloid Leukemia
- PMID: 39273395
- PMCID: PMC11394928
- DOI: 10.3390/ijms25179448
Enhancing Therapeutic Efficacy of FLT3 Inhibitors with Combination Therapy for Treatment of Acute Myeloid Leukemia
Abstract
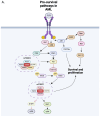
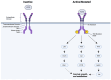
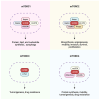
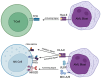
FMS-like tyrosine kinase 3 (FLT3) mutations are genetic changes found in approximately thirty percent of patients with acute myeloid leukemia (AML). FLT3 mutations in AML represent a challenging clinical scenario characterized by a high rate of relapse, even after allogeneic hematopoietic stem cell transplantation (allo-HSCT). The advent of FLT3 tyrosine kinase inhibitors (TKIs), such as midostaurin and gilteritinib, has shown promise in achieving complete remission. However, a substantial proportion of patients still experience relapse following TKI treatment, necessitating innovative therapeutic strategies. This review critically addresses the current landscape of TKI treatments for FLT3+ AML, with a particular focus on gilteritinib. Gilteritinib, a highly selective FLT3 inhibitor, has demonstrated efficacy in targeting the mutant FLT3 receptor, thereby inhibiting aberrant signaling pathways that drive leukemic proliferation. However, monotherapy with TKIs may not be sufficient to eradicate AML blasts. Specifically, we provide evidence for integrating gilteritinib with mammalian targets of rapamycin (mTOR) inhibitors and interleukin-15 (IL-15) complexes. The combination of gilteritinib, mTOR inhibitors, and IL-15 complexes presents a compelling strategy to enhance the eradication of AML blasts and enhance NK cell killing, offering a potential for improved patient outcomes.
Keywords: NK cells; acute myeloid leukemia (AML); gilteritinib; mTOR inhibitors; rapamycin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Pelcovits A., Niroula R. Acute Myeloid Leukemia: A review. RIMedJ. 2020;103:38–40. - PubMed
-
- Grimwade D., Walker H., Oliver F., Wheatley K., Harrison C., Harrison G., Rees J., Hann I., Stevens R., Burnett A., et al. The Importance of Diagnostic Cytogenetics on Outcome in AML: Analysis of 1,612 Patients Entered into the MRC AML 10 Trial. Blood. 1998;92:2322–2333. doi: 10.1182/blood.V92.7.2322. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

