Functions and Clinical Applications of Extracellular Vesicles in TH2 Cell-Mediated Airway Inflammatory Diseases: A Review
- PMID: 39273399
- PMCID: PMC11394744
- DOI: 10.3390/ijms25179455
Functions and Clinical Applications of Extracellular Vesicles in TH2 Cell-Mediated Airway Inflammatory Diseases: A Review
Abstract
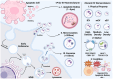
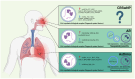
Type 2 airway inflammation (T2AI), driven by type 2 innate lymphoid and CD4+ T helper 2 cells, leads to various diseases and conditions, such as chronic rhinosinusitis with nasal polyps, allergic rhinitis, and asthma. Emerging evidence suggests the involvement of extracellular vesicles (EVs) in these diseases. In this review, we describe the immunological T2AI pathogenic mechanisms, outline EV characteristics, and highlight their applications in the diagnosis and treatment of T2AI. An extensive literature search was conducted using appropriate strategies to identify relevant articles from various online databases. EVs in various biological samples showed disease-specific characteristics for chronic rhinosinusitis with nasal polyps, allergic rhinitis, and asthma, with some demonstrating therapeutic effects against these conditions. However, most studies have been limited to in vitro and animal models, highlighting the need for further clinical research on the diagnostic and therapeutic applications of EVs.
Keywords: allergic rhinitis; asthma; chronic rhinosinusitis with nasal polyp; extracellular vesicles; type 2 inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Fokkens W.J., Lund V.J., Hopkins C., Hellings P.W., Kern R., Reitsma S., Toppila-Salmi S., Bernal-Sprekelsen M., Alobid J.M.I., Anselmo-Lima Terezinha W., et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58((Suppl. S29)):1–464. doi: 10.4193/Rhin20.401. - DOI - PubMed
-
- Reddel H.K., Bacharier L.B., Bateman E.D., Brightling C.E., Brusselle G.G., Buhl R., Cruz A.A., Duijts L., Drazen J.M., FitzGerald J.M., et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Am. J. Respir. Crit. Care Med. 2022;205:17–35. doi: 10.1164/rccm.202109-2205PP. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 2017R1A2B2003575/Ministry of Science and Technology and the Ministry of Science, ICT & Future Planning
- NRF-2020R1A2C1006398/Basic Science Research Program of the National Research Foundation of Korea
- 2020R1C1C1012288/the Ministry of Science and ICT
- IITP-2024-2020-0-01819/Korea, under the ICT Creative Consilience program
- HI17C0387/Korea Health Technology R&D Project
LinkOut - more resources
Full Text Sources
Research Materials

