Mitochondrial Plasticity and Glucose Metabolic Alterations in Human Cancer under Oxidative Stress-From Viewpoints of Chronic Inflammation and Neutrophil Extracellular Traps (NETs)
- PMID: 39273403
- PMCID: PMC11395599
- DOI: 10.3390/ijms25179458
Mitochondrial Plasticity and Glucose Metabolic Alterations in Human Cancer under Oxidative Stress-From Viewpoints of Chronic Inflammation and Neutrophil Extracellular Traps (NETs)
Abstract
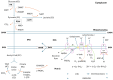
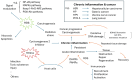
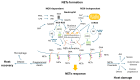
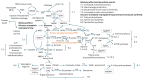
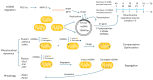
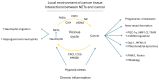
Oxidative stress elicited by reactive oxygen species (ROS) and chronic inflammation are involved both in deterring and the generation/progression of human cancers. Exogenous ROS can injure mitochondria and induce them to generate more endogenous mitochondrial ROS to further perpetuate the deteriorating condition in the affected cells. Dysfunction of these cancer mitochondria may possibly be offset by the Warburg effect, which is characterized by amplified glycolysis and metabolic reprogramming. ROS from neutrophil extracellular traps (NETs) are an essential element for neutrophils to defend against invading pathogens or to kill cancer cells. A chronic inflammation typically includes consecutive NET activation and tissue damage, as well as tissue repair, and together with NETs, ROS would participate in both the destruction and progression of cancers. This review discusses human mitochondrial plasticity and the glucose metabolic reprogramming of cancer cells confronting oxidative stress by the means of chronic inflammation and neutrophil extracellular traps (NETs).
Keywords: Warburg effect; inflammation; mitochondrial plasticity; neutrophil; neutrophil extracellular traps (NETs); oxidative stress.
Conflict of interest statement
Author Po Chen was employed by the company Cancer Free Biotech and author Chang-Youh Tsai was employed by the company Clinical Trial Center . The remaining authors declare that the re-search was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cooper G.M. The Cell: A Molecular Approach. 2nd ed. Sinauer Associates; Sunderland, MA, USA: 2000. [(accessed on 20 August 2024)]. The Mechanism of Oxidative Phosphorylation. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9885/
-
- Nakrani M.N., Wineland R.H., Anjum F. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Physiology, Glucose Metabolism. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

